Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa
- PMID: 31534016
- PMCID: PMC7199879
- DOI: 10.1126/scitranslmed.aax1880
Immune correlates of the Thai RV144 HIV vaccine regimen in South Africa
Abstract
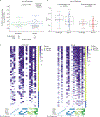
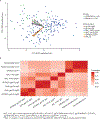
One of the most successful HIV vaccines to date, the RV144 vaccine tested in Thailand, demonstrated correlates of protection including cross-clade V1V2 immunoglobulin G (IgG) breadth, Env-specific CD4+ T cell polyfunctionality, and antibody-dependent cellular cytotoxicity (ADCC) in vaccinees with low IgA binding. The HIV Vaccine Trials Network (HVTN) 097 trial evaluated this vaccine regimen in South Africa, where clade C HIV-1 predominates. We compared cellular and humoral responses at peak and durability immunogenicity time points in HVTN 097 and RV144 vaccinee samples, and evaluated vaccine-matched and cross-clade immune responses. At peak immunogenicity, HVTN 097 vaccinees exhibited significantly higher cellular and humoral immune responses than RV144 vaccinees. CD4+ T cell responses were more frequent in HVTN 097 irrespective of age and sex, and CD4+ T cell Env-specific functionality scores were higher in HVTN 097. Env-specific CD40L+ CD4+ T cells were more common in HVTN 097, with individuals having this pattern of expression demonstrating higher median antibody responses to HIV-1 Env. IgG and IgG3 binding antibody rates and response magnitude to gp120 vaccine- and V1V2 vaccine-matched antigens were higher or comparable in HVTN 097 than in RV144 ADCC, and ADCP functional antibody responses were elicited in HVTN 097. Env-specific IgG and CD4+ Env responses declined significantly over time in both trials. Overall, cross-clade immune responses associated with protection were better than expected in South Africa, suggesting wider applicability of this regimen.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures






References
-
- UNAIDS, “Global AIDS Update,” (2016).
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, Investigators M-T, Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361, 2209–2220 (2009). - PubMed
-
- Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, Paris RM, Chiu J, Adams E, Francis D, Gurunathan S, Tartaglia J, Gilbert P, Stablein D, Michael NL, Kim JH, Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 12, 531–537 (2012). - PMC - PubMed
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH, Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366, 1275–1286 (2012). - PMC - PubMed
-
- Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC, Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 8, e75665 (2013). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

