Tracking the Fate of Endogenous Retrovirus Segregation in Wild and Domestic Cats
- PMID: 31534037
- PMCID: PMC6880155
- DOI: 10.1128/JVI.01324-19
Tracking the Fate of Endogenous Retrovirus Segregation in Wild and Domestic Cats
Abstract
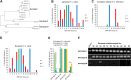
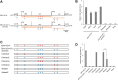
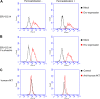
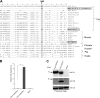
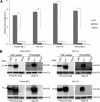
Endogenous retroviruses (ERVs) of domestic cats (ERV-DCs) are one of the youngest feline ERV groups in domestic cats (Felis silvestris catus); some members are replication competent (ERV-DC10, ERV-DC18, and ERV-DC14), produce the antiretroviral soluble factor Refrex-1 (ERV-DC7 and ERV-DC16), or can generate recombinant feline leukemia virus (FeLV). Here, we investigated ERV-DC in European wildcats (Felis silvestris silvestris) and detected four loci: ERV-DC6, ERV-DC7, ERV-DC14, and ERV-DC16. ERV-DC14 was detected at a high frequency in European wildcats; however, it was replication defective due to a single G → A nucleotide substitution, resulting in an E148K substitution in the ERV-DC14 envelope (Env). This mutation results in a cleavage-defective Env that is not incorporated into viral particles. Introduction of the same mutation into feline and murine infectious gammaretroviruses resulted in a similar Env dysfunction. Interestingly, the same mutation was found in an FeLV isolate from naturally occurring thymic lymphoma and a mouse ERV, suggesting a common mechanism of virus inactivation. Refrex-1 was present in European wildcats; however, ERV-DC16, but not ERV-DC7, was unfixed in European wildcats. Thus, Refrex-1 has had an antiviral role throughout the evolution of the genus Felis, predating cat exposure to feline retroviruses. ERV-DC sequence diversity was present across wild and domestic cats but was locus dependent. In conclusion, ERVs have evolved species-specific phenotypes through the interplay between ERVs and their hosts. The mechanism of viral inactivation may be similar irrespective of the evolutionary history of retroviruses. The tracking of ancestral retroviruses can shed light on their roles in pathogenesis and host-virus evolution.IMPORTANCE Domestic cats (Felis silvestris catus) were domesticated from wildcats approximately 9,000 years ago via close interaction between humans and cats. During cat evolution, various exogenous retroviruses infected different cat lineages and generated numerous ERVs in the host genome, some of which remain replication competent. Here, we detected several ERV-DC loci in Felis silvestris silvestris Notably, a species-specific single nucleotide polymorphism in the ERV-DC14 env gene, which results in a replication-defective product, is highly prevalent in European wildcats, unlike the replication-competent ERV-DC14 that is commonly present in domestic cats. The presence of the same lethal mutation in the env genes of both FeLV and murine ERV provides a common mechanism shared by endogenous and exogenous retroviruses by which ERVs can be inactivated after endogenization. The antiviral role of Refrex-1 predates cat exposure to feline retroviruses. The existence of two ERV-DC14 phenotypes provides a unique model for understanding both ERV fate and cat domestication.
Keywords: ERV-DC; FeLV; Felis; Fv-4; MuLV; domestic cat; domestication; endogenous retrovirus; evolution; wildcat.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- Pontius JU, Mullikin JC, Smith DR, Agencourt Sequencing Team, Lindblad-Toh K, Gnerre S, Clamp M, Chang J, Stephens R, Neelam B, Volfovsky N, Schäffer AA, Agarwala R, Narfström K, Murphy WJ, Giger U, Roca AL, Antunes A, Menotti-Raymond M, Yuhki N, Pecon-Slattery J, Johnson WE, Bourque G, Tesler G, NISC Comparative Sequencing Program, O’Brien SJ. 2007. Initial sequence and comparative analysis of the cat genome. Genome Res 17:1675–1689. doi: 10.1101/gr.6380007. - DOI - PMC - PubMed
-
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, et al. 2002. Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562. doi: 10.1038/nature01262. - DOI - PubMed
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann Y, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, et al. 2001. Initial sequencing and analysis of the human genome. Nature 409:860–921. doi: 10.1038/35057062. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

