Lowering circulating apolipoprotein E levels improves aged bone fracture healing
- PMID: 31534056
- PMCID: PMC6795296
- DOI: 10.1172/jci.insight.129144
Lowering circulating apolipoprotein E levels improves aged bone fracture healing
Abstract
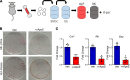
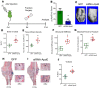
Age is a well-established risk factor for impaired bone fracture healing. Here, we identify a role for apolipoprotein E (ApoE) in age-associated impairment of bone fracture healing and osteoblast differentiation, and we investigate the mechanism by which ApoE alters these processes. We identified that, in both humans and mice, circulating ApoE levels increase with age. We assessed bone healing in WT and ApoE-/- mice after performing tibial fracture surgery: bone deposition was higher within fracture calluses from ApoE-/- mice. In vitro recombinant ApoE (rApoE) treatment of differentiating osteoblasts decreased cellular differentiation and matrix mineralization. Moreover, this rApoE treatment decreased osteoblast glycolytic activity while increasing lipid uptake and fatty acid oxidation. Using parabiosis models, we determined that circulating ApoE plays a strong inhibitory role in bone repair. Using an adeno-associated virus-based siRNA system, we decreased circulating ApoE levels in 24-month-old mice and demonstrated that, as a result, fracture calluses from these aged mice displayed enhanced bone deposition and mechanical strength. Our results demonstrate that circulating ApoE as an aging factor inhibits bone fracture healing by altering osteoblast metabolism, thereby identifying ApoE as a new therapeutic target for improving bone repair in the elderly.
Keywords: Bone Biology; Bone disease; Osteoclast/osteoblast biology.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

