Different Influences of Statin Treatment in Preventing At-Risk Stroke Subtypes: A Post Hoc Analysis of J-STARS
- PMID: 31534062
- PMCID: PMC7242230
- DOI: 10.5551/jat.50518
Different Influences of Statin Treatment in Preventing At-Risk Stroke Subtypes: A Post Hoc Analysis of J-STARS
Abstract
Aims: To understand the different influences of statins on the incidence rate of each stroke subtype in association with low-density lipoprotein (LDL) cholesterol levels, we performed a post hoc analysis on the data from the Japan Statin Treatment Against Recurrent Stroke (J-STARS) study.
Methods: Subjects (n=1,578) were divided into three groups according to their mean postrandomized LDL cholesterol level (<100, 100-120, and ≥ 120 mg/dL) until the last observation before the event or the end of follow-up. A Cox proportional hazard model for time to events was used for calculating adjusted hazard ratios, 95% confidence intervals, and the trend tests.
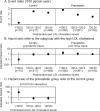
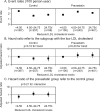
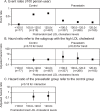
Results: The event rates for atherothrombotic stroke did not decrease in accordance with the postrandomized LDL cholesterol level subgroups of either the control or the pravastatin group (p=0.15 and 0.33 for the trend, respectively). In the control group, however, no atherothrombotic stroke event was observed in the subgroup of the low postrandomized LDL cholesterol level (less than 100 mg/dL). The event rates for atherothrombotic stroke were lower in the middle postrandomized LDL cholesterol level subgroup (100-120 mg/dL) of the pravastatin group than that of the control group. The event rates for lacunar stroke decreased in the lower postrandomized LDL cholesterol level subgroup of the control group but not of the pravastatin group (p=0.004 and 0.06 for the trend, respectively).
Conclusions: Statins showed different influences on the risks of atherothromobotic and lacunar stroke according to postrandomized LDL cholesterol levels.
Keywords: Atherothrombotic stroke; Cholesterol; Intracranial hemorrhage; Lacunar stroke; Statin.
Conflict of interest statement
Dr. Kitagawa reports personal fees from Daiichi Sankyo, during the conduct of the study; personal fees from Bayer Inc., Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, and Sanofi, outside the submitted work; and grants from Daiichi Sankyo during the conduct of the study; grants from Bayer Inc., Takeda Pharmaceutical, Nippon Boehringer Ingelheim, Kyowa Hakko Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, and Sanofi, outside the submitted work.
Dr. Maruyama reports grants from Grants-in-Aid for Scientific Research, Eisai, Pfizer, Otsuka Pharmaceutical, Shionogi, Sumitomo Dainippon Pharma, Nihon Medi-Physics, Bayer, MSD, Daiichi Sankyo, Sanofi, and Astellas Pharma, outside the submitted work.
Dr. Minematsu reports personal fees from Bayer Yakuhin, Otsuka Pharmaceutical, Boehringer-Ingelhaeim, AstraZeneca, Pfizer, Mitsubishi Tanabe Pharma Corporation, Japan Stryker, Daiichi Sankyo, Astellas Pharma, Nippon Chemiphar, Fuji Film RI Pharma, CSL Behring, Medicos Hirata, EPS Corporation, HEALIOS K.K., and T-PEC Corporation, outside the submitted work.
Dr. Uchiyama reports personal fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Sanofi, Daiichi Sankyo, Dainippon Sumitomo, Astellas Pharma, AstraZeneca, Sannwa Kagaku, Shionogi, Mitsubishi Tanabe, and Pfizer, outside the submitted work.
Dr. Matsumoto reports personal fees from Kowa Pharmaceutical Co Ltd, Takeda Pharmaceutical Co Ltd, Bayer Yakuhin, Ltd, Sanofi KK, Daiichi Sankyo Co Ltd, Otsuka Pharmaceutical Co Ltd, Astellas Pharma Inc, Astra Zeneca KK, Mochida Pharmaceutical Co Ltd, Sumitomo Dainippon Pharma Co Ltd, Amgen Astellas BioPharma KK, Eisai Co Ltd, and Pfizer Japan Inc, outside the submitted work.
Ms. Nakagawa and Mr. Kagimura are employed at the Translational Research Informatics Center, which contracted with the J-STARS office as a data center and received a data center fee.
The other authors declare that they have no conflicts of interest.
Figures

References
-
- Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, Hennerici M, Simunovic L, Zivin JA, Welch KM, Investigators S : Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2007; 38: 3198-3204 - PubMed
-
- Sillesen H, Amarenco P, Hennerici MG, Callahan A, Goldstein LB, Zivin J, Messig M, Welch KM, Stroke Prevention by Aggressive Reduction in Cholesterol Levels I : Atorvastatin reduces the risk of cardiovascular events in patients with carotid atherosclerosis: a secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke, 2008; 39: 3297-3302 - PubMed
-
- Amarenco P, Benavente O, Goldstein LB, Callahan A, 3rd, Sillesen H, Hennerici MG, Gilbert S, Rudolph AE, Simunovic L, Zivin JA, Welch KM: Results of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial by stroke subtypes. Stroke, 2009; 40: 1405-1409 - PubMed
-
- Kitagawa K, Hosomi N, Nagai Y, Kagimura T, Ohtsuki T, Origasa H, Minematsu K, Uchiyama S, Nakamura M, Matsumoto M, J-STARS Investigators : Reduction in high-sensitivity C-reactive protein levels in patients with ischemic stroke by statin treatment: Hs-CRP Sub-Study in J-STARS. J Atheroscler Thromb, 2017; 24: 1039-1047 - PMC - PubMed

