Novelty and Novel Objects Increase c-Fos Immunoreactivity in Mossy Cells in the Mouse Dentate Gyrus
- PMID: 31534449
- PMCID: PMC6732597
- DOI: 10.1155/2019/1815371
Novelty and Novel Objects Increase c-Fos Immunoreactivity in Mossy Cells in the Mouse Dentate Gyrus
Abstract
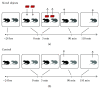
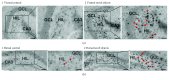
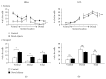
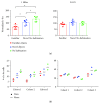
The dentate gyrus (DG) and its primary cell type, the granule cell (GC), are thought to be critical to many cognitive functions. A major neuronal subtype of the DG is the hilar mossy cell (MC). MCs have been considered to play an important role in cognition, but in vivo studies to understand the activity of MCs during cognitive tasks are challenging because the experiments usually involve trauma to the overlying hippocampus or DG, which kills hilar neurons. In addition, restraint typically occurs, and MC activity is reduced by brief restraint stress. Social isolation often occurs and is potentially confounding. Therefore, we used c-fos protein expression to understand when MCs are active in vivo in socially housed adult C57BL/6 mice in their home cage. We focused on c-fos protein expression after animals explored novel objects, based on previous work which showed that MCs express c-fos protein readily in response to a novel housing location. Also, MCs are required for the training component of the novel object location task and novelty-encoding during a food-related task. GluR2/3 was used as a marker of MCs. The results showed that MC c-fos protein is greatly increased after exposure to novel objects, especially in ventral DG. We also found that novel objects produced higher c-fos levels than familiar objects. Interestingly, a small subset of neurons that did not express GluR2/3 also increased c-fos protein after novel object exposure. In contrast, GCs appeared relatively insensitive. The results support a growing appreciation of the role of the DG in novelty detection and novel object recognition, where hilar neurons and especially MCs are very sensitive.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

