Focused ultrasound activates voltage-gated calcium channels through depolarizing TRPC1 sodium currents in kidney and skeletal muscle
- PMID: 31534500
- PMCID: PMC6735402
- DOI: 10.7150/thno.33876
Focused ultrasound activates voltage-gated calcium channels through depolarizing TRPC1 sodium currents in kidney and skeletal muscle
Erratum in
-
Erratum: Focused ultrasound activates voltage-gated calcium channels through depolarizing TRPC1 sodium currents in kidney and skeletal muscle.Theranostics. 2022 Jan 1;12(3):1341. doi: 10.7150/thno.69908. eCollection 2022. Theranostics. 2022. PMID: 35154493 Free PMC article.
Abstract
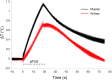
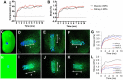
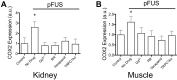
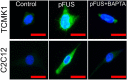
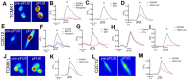
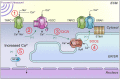
Pulsed focused ultrasound (pFUS) technology is being developed for clinical neuro/immune modulation and regenerative medicine. Biological signal transduction of pFUS forces can require mechanosensitive or voltage-gated plasma membrane ion channels. Previous studies suggested pFUS is capable of activating either channel type, but their mechanistic relationship remains ambiguous. We demonstrated pFUS bioeffects increased mesenchymal stem cell tropism (MSC) by altering molecular microenvironments through cyclooxygenase-2 (COX2)-dependent pathways. This study explored specific relationships between mechanosensitive and voltage-gated Ca2+ channels (VGCC) to initiate pFUS bioeffects that increase stem cell tropism. Methods: Murine kidneys and hamstring were given pFUS (1.15 or 1.125 MHz; 4MPa peak rarefactional pressure) under ultrasound or magnetic resonance imaging guidance. Cavitation and tissue displacement were measure by hydrophone and ultrasound radiofrequency data, respectively. Elastic modeling was performed from displacement measurements. COX2 expression and MSC tropism were evaluated in the presence of pharmacological ion channel inhibitors or in transient-receptor-potential-channel-1 (TRPC1)-deficient mice. Immunohistochemistry and co-immunoprecipitation examined physical channel relationships. Fluorescent ionophore imaging of cultured C2C12 muscle cells or TCMK1 kidney cells probed physiological interactions. Results: pFUS induced tissue deformations resulting in kPa-scale forces suggesting mechanical activation of pFUS-induced bioeffects. Inhibiting VGCC or TRPC1 in vivo blocked pFUS-induced COX2 upregulation and MSC tropism to kidneys and muscle. A TRPC1/VGCC complex was observed in plasma membranes. VGCC or TRPC1 suppression blocked pFUS-induced Ca2+ transients in TCMK1 and C2C12 cells. Additionally, Ca2+ transients were blocked by reducing transmembrane Na+ potentials and observed Na+ transients were diminished by genetic TRPC1 suppression. Conclusion: This study suggests that pFUS acoustic radiation forces mechanically activate a Na+-containing TRPC1 current upstream of VGCC rather than directly opening VGCC. The electrogenic function of TRPC1 provides potential mechanistic insight into other pFUS techniques for physiological modulation and optimization strategies for clinical implementation.
Keywords: Acoustic radiation force; Calcium signaling; Cavitation; Depolarization; Mechanotransduction; Transient Receptor Potential Channel 1; Voltage-Gated Calcium Channels; focused ultrasound.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

