Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice
- PMID: 31534531
- PMCID: PMC6735367
- DOI: 10.7150/thno.33872
Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice
Abstract
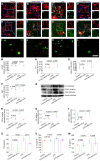
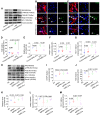
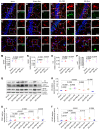
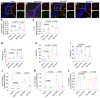
Mesenchymal stem cell-derived exosomes (MSC-Exo) have robust anti-inflammatory effects in the treatment of neurological diseases such as epilepsy, stroke, or traumatic brain injury. While astrocytes are thought to be mediators of these effects, their precise role remains poorly understood. To address this issue, we investigated the putative therapeutic effects and mechanism of MSC-Exo on inflammation-induced alterations in astrocytes. Methods: Lipopolysaccharide (LPS)-stimulated hippocampal astrocytes in primary culture were treated with MSC-Exo, which were also administered in pilocarpine-induced status epilepticus (SE) mice. Exosomal integration, reactive astrogliosis, inflammatory responses, calcium signaling, and mitochondrial membrane potentials (MMP) were monitored. To experimentally probe the molecular mechanism of MSC-Exo actions on the inflammation-induced astrocytic activation, we inhibited the nuclear factor erythroid-derived 2, like 2 (Nrf2, a key mediator in neuroinflammation and oxidative stress) by sgRNA (in vitro) or ML385 (Nrf2 inhibitor) in vivo. Results: MSC-Exo were incorporated into hippocampal astrocytes as well as attenuated reactive astrogliosis and inflammatory responses in vitro and in vivo. Also, MSC-Exo ameliorated LPS-induced aberrant calcium signaling and mitochondrial dysfunction in culture, and SE-induced learning and memory impairments in mice. Furthermore, the putative therapeutic effects of MSC-Exo on inflammation-induced astrocytic activation (e.g., reduced reactive astrogliosis, NF-κB deactivation) were weakened by Nrf2 inhibition. Conclusions: Our results show that MSC-Exo ameliorate inflammation-induced astrocyte alterations and that the Nrf2-NF-κB signaling pathway is involved in regulating astrocyte activation in mice. These data suggest the promising potential of MSC-Exo as a nanotherapeutic agent for the treatment of neurological diseases with hippocampal astrocyte alterations.
Keywords: MSC-Exo; Nanotherapy; Nrf2-NF-κB signaling; astrocyte alterations; calcium signaling.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W. et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33:7008–18. - PubMed
-
- Si YL, Zhao YL, Hao HJ, Fu XB, Han WD MSCs. Biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10:93–103. - PubMed
-
- Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009–16. - PubMed
-
- Shi Y, Wang Y, Li Q, Liu K, Hou J, Shao C. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 2018;14:493–507. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

