Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs
- PMID: 31534532
- PMCID: PMC6735380
- DOI: 10.7150/thno.33959
Galectin 3 protects from cisplatin-induced acute kidney injury by promoting TLR-2-dependent activation of IDO1/Kynurenine pathway in renal DCs
Abstract
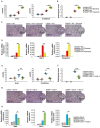
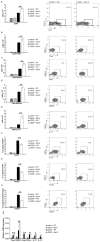
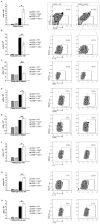
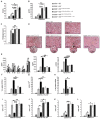
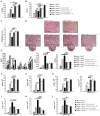
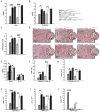
Strategies targeting cross-talk between immunosuppressive renal dendritic cells (DCs) and T regulatory cells (Tregs) may be effective in treating cisplatin (CDDP)-induced acute kidney injury (AKI). Galectin 3 (Gal-3), expressed on renal DCs, is known as a crucial regulator of immune response in the kidneys. In this study, we investigated the role of Gal-3 for DCs-mediated expansion of Tregs in the attenuation of CDDP-induced AKI. Methods: AKI was induced in CDDP-treated wild type (WT) C57BL/6 and Gal-3 deficient (Gal-3-/-) mice. Biochemical, histological analysis, enzyme-linked immunosorbent assay (ELISA), immunohistochemistry, real-time PCR, magnetic cell sorting, flow cytometry and intracellular staining of renal-infiltrated immune cells were used to determine the differences between CDDP-treated WT and Gal-3-/- mice. Newly synthesized selective inhibitor of Gal-3 (Davanat) was used for pharmacological inhibition of Gal-3. Recombinant Gal-3 was used to demonstrate the effects of exogenously administered soluble Gal-3 on AKI progression. Pam3CSK4 was used for activation of Toll-like receptor (TLR)-2 in DCs. Cyclophosphamide or anti-CD25 antibody were used for the depletion of Tregs. 1-Methyl Tryptophan (1-MT) was used for pharmacological inhibition of Indoleamine 2,3-dioxygenase-1 (IDO1) in TLR-2-primed DCs which were afterwards used in passive transfer experiments. Results: CDDP-induced nephrotoxicity was significantly more aggravated in Gal-3-/- mice. Significantly reduced number of immunosuppressive TLR-2 and IDO1-expressing renal DCs, lower serum levels of KYN, decreased presence of IL-10-producing Tregs and significantly higher number of inflammatory IFN-γ and IL-17-producing neutrophils, Th1 and Th17 cells were observed in the CDDP-injured kidneys of Gal-3-/- mice. Pharmacological inhibitor of Gal-3 aggravated CDDP-induced AKI in WT animals while recombinant Gal-3 attenuated renal injury and inflammation in CDDP-treated Gal-3-/- mice. CDDP-induced apoptosis, driven by Bax and caspase-3, was aggravated in Gal-3-/- animals and in WT mice that received Gal-3 inhibitor (CDDP+Davanat-treated mice). Recombinant Gal-3 managed to completely attenuate CDDP-induced apoptosis in CDDP-injured kidneys of Gal-3-/- mice. Genetic deletion as well as pharmacological inhibition of Gal-3 in renal DCs remarkably reduced TLR-2-dependent activation of IDO1/KYN pathway in these cells diminishing their capacity to prevent transdifferentiation of Tregs in inflammatory Th1 and Th17 cells. Additionally, Tregs generated by Gal-3 deficient DCs were not able to suppress production of IFN-γ and IL-17 in activated neutrophils. TLR-2-primed DCs significantly enhanced capacity of Tregs for attenuation of CDDP-induced AKI and inflammation and expression of Gal-3 on TLR-2-primed DCs was crucially important for their capacity to enhance nephroprotective and immunosuppressive properties of Tregs. Adoptive transfer of TLR-2-primed WTDCs significantly expanded Tregs in the kidneys of CDDP-treated WT and Gal-3-/- recipients resulting in the suppression of IFN-γ and IL-17-driven inflammation and alleviation of AKI. Importantly, this phenomenon was not observed in CDDP-treated WT and Gal-3-/- recipients of TLR-2-primed Gal-3-/-DCs. Gal-3-dependent nephroprotective and immunosuppressive effects of renal DCs was due to the IDO1-induced expansion of renal Tregs since either inhibition of IDO1 activity in TLR-2-primed DCs or depletion of Tregs completely diminished DCs-mediated attenuation of CDDP-induced AKI. Conclusions: Gal-3 protects from CDDP-induced AKI by promoting TLR-2-dependent activation of IDO1/KYN pathway in renal DCs resulting in increased expansion of immunosuppressive Tregs in injured kidneys. Activation of Gal-3:TLR-2:IDO1 pathway in renal DCs should be further explored as new therapeutic approach for DC-based immunosuppression of inflammatory renal diseases.
Keywords: 3-dioxygenase-1; Galectin 3; Indoleamine 2; Toll-like receptor-2; cisplatin-induced acute kidney injury; renal dendritic cells.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. - PubMed
-
- Andrade-Silva M, Cenedeze MA, Perandini LA. et al. TLR2 and TLR4 play opposite role in autophagy associated with cisplatin-induced acute kidney injury. Clin Sci (Lond) 2018;132:1725–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

