Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation
- PMID: 31534619
- PMCID: PMC6732632
- DOI: 10.1155/2019/2432416
Sesamin Enhances Nrf2-Mediated Protective Defense against Oxidative Stress and Inflammation in Colitis via AKT and ERK Activation
Abstract
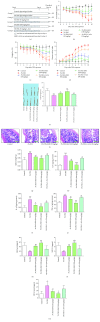
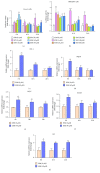
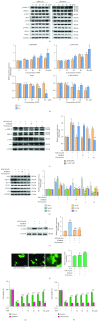
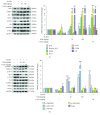
Ulcerative colitis (UC) is a major form of inflammatory bowel disease (IBD) with high incidence and prevalence in many countries. Patients with UC usually suffer from a lifetime of debilitating physical symptoms. Therefore, developing effective therapeutic strategy that can manage this disease better and improve patients' life quality is in urgent need. Sesamin (SSM) is a lignan derived from sesame seeds. In this study, the protective effect of SSM against UC and the underlying mechanism were investigated in vitro and in vivo. Our data showed that SSM protected Caco-2 cells from H2O2-induced oxidative stress injury via GSH-mediated scavenging of reactive oxygen species (ROS). Dual luciferase reporter assay showed that the transcriptional activity of nuclear factor erythroid-related factor 2 (Nrf2) was significantly increased by SSM, and the ability of SSM to activate Nrf2-targeted genes was further confirmed in Caco-2 cells using western blot and quantitative real-time PCR (qRT-PCR). In contrast, Nrf2 knockdown abolished the protective effect of SSM. Additionally, we found that SSM also activated advanced protein kinase B (AKT) and extracellular signal-regulated kinase (ERK) in Caco-2 cells, while either AKT or ERK inhibition can prevent SSM-mediated nuclear translocation of Nrf2. Furthermore, SSM displayed a better protective effect against dextran sulfate sodium- (DSS-) induced UC compared with 5-aminosalicylic acid (5-ASA) in C57BL/6 mice. The enhanced Nrf2 signaling and activated AKT/ERK were also observed in the colon of mice after SSM administration. These results first demonstrate the protective effect of SSM against UC and indicate that the effect is associated with AKT/ERK activation and subsequent Nrf2 signaling enhancement. This study provides a new insight into the medicinal value of SSM and proposes it as a new natural nutrition for better managing the symptoms of UC.
Conflict of interest statement
There are no potential conflicts of interest in this study.
Figures


Similar articles
-
The cytoprotective effects of 7,8-dihydroxyflavone against oxidative stress are mediated by the upregulation of Nrf2-dependent HO-1 expression through the activation of the PI3K/Akt and ERK pathways in C2C12 myoblasts.Int J Mol Med. 2015 Aug;36(2):501-10. doi: 10.3892/ijmm.2015.2256. Epub 2015 Jun 22. Int J Mol Med. 2015. PMID: 26096841
-
Hepatocyte-protective effect of nectandrin B, a nutmeg lignan, against oxidative stress: Role of Nrf2 activation through ERK phosphorylation and AMPK-dependent inhibition of GSK-3β.Toxicol Appl Pharmacol. 2016 Sep 15;307:138-149. doi: 10.1016/j.taap.2016.08.003. Epub 2016 Aug 7. Toxicol Appl Pharmacol. 2016. PMID: 27511913
-
Lico A Enhances Nrf2-Mediated Defense Mechanisms against t-BHP-Induced Oxidative Stress and Cell Death via Akt and ERK Activation in RAW 264.7 Cells.Oxid Med Cell Longev. 2015;2015:709845. doi: 10.1155/2015/709845. Epub 2015 Oct 20. Oxid Med Cell Longev. 2015. PMID: 26576227 Free PMC article.
-
Does insulin bolster antioxidant defenses via the extracellular signal-regulated kinases-protein kinase B-nuclear factor erythroid 2 p45-related factor 2 pathway?Antioxid Redox Signal. 2012 May 15;16(10):1061-70. doi: 10.1089/ars.2011.4460. Epub 2012 Feb 7. Antioxid Redox Signal. 2012. PMID: 22149292 Review.
-
Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis.Biochem Pharmacol. 2008 Dec 1;76(11):1485-9. doi: 10.1016/j.bcp.2008.07.017. Epub 2008 Jul 23. Biochem Pharmacol. 2008. PMID: 18694732 Free PMC article. Review.
Cited by
-
Sesamin Induces the Transdifferentiation of Type II Alveolar Epithelial Cells via AnnexinA1 and TRPV1.Lung. 2023 Feb;201(1):65-77. doi: 10.1007/s00408-023-00598-7. Epub 2023 Feb 3. Lung. 2023. PMID: 36735045
-
Icariside II Attenuates Methamphetamine-Induced Neurotoxicity and Behavioral Impairments via Activating the Keap1-Nrf2 Pathway.Oxid Med Cell Longev. 2022 Mar 28;2022:8400876. doi: 10.1155/2022/8400876. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35387263 Free PMC article.
-
4-hydroxysesamin protects rat with right ventricular failure due to pulmonary hypertension by inhibiting JNK/p38 MAPK signaling.Aging (Albany NY). 2024 May 8;16(9):8142-8154. doi: 10.18632/aging.205808. Epub 2024 May 8. Aging (Albany NY). 2024. PMID: 38728253 Free PMC article.
-
Sesamin-mediated high expression of BECN2 ameliorates cartilage endplate degeneration by reducing autophagy and inflammation.Aging (Albany NY). 2024 Jan 26;16(2):1145-1160. doi: 10.18632/aging.205386. Epub 2024 Jan 26. Aging (Albany NY). 2024. PMID: 38284902 Free PMC article.
-
Mulberry Anthocyanins Ameliorate DSS-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Modulating Gut Microbiota.Antioxidants (Basel). 2022 Aug 27;11(9):1674. doi: 10.3390/antiox11091674. Antioxidants (Basel). 2022. PMID: 36139747 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

