Antiobesity, Regulation of Lipid Metabolism, and Attenuation of Liver Oxidative Stress Effects of Hydroxy- α-sanshool Isolated from Zanthoxylum bungeanum on High-Fat Diet-Induced Hyperlipidemic Rats
- PMID: 31534622
- PMCID: PMC6732614
- DOI: 10.1155/2019/5852494
Antiobesity, Regulation of Lipid Metabolism, and Attenuation of Liver Oxidative Stress Effects of Hydroxy- α-sanshool Isolated from Zanthoxylum bungeanum on High-Fat Diet-Induced Hyperlipidemic Rats
Abstract
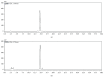
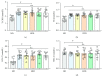
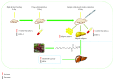
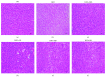
Zanthoxylum bungeanum is a traditional Chinese medicine (TCM) used to relieve pain, dispel dampness, stop diarrhea, and prevent itching. The aim of this study was to investigate the antiobesity and hypolipidemic effects of hydroxy-α-sanshool (HAS) isolated from Z. bungeanum on hyperlipidemic rats. Wistar rats (n = 48) were randomly divided into six groups: (1) normal diet rats (ND), (2) high-fat diet- (HFD-) treated rats, (3) HFD+fenofibrate-treated rats (HFD+FNB), (4) HFD+low dose of HAS-treated rats (HFD+LD, 9 mg/kg), (5) HFD+middle dose of HAS-treated rats (HFD+MD, 18 mg/kg), and (6) HFD+high dose of HAS-treated rats (HFD+HD, 36 mg/kg). The body weight and food intake of the rats were recorded during the treatment period. After 4 weeks of HAS treatment, abdominal adipose tissues were observed and total cholesterol (T-CHO), triglycerides (TG), high-density lipoprotein (HDL) cholesterol (HDL-C), and low-density lipoprotein (LDL) cholesterol (LDL-C) of serum and liver tissues were determined. Furthermore, histochemical examinations using oil red O and hematoxylin-eosin staining (H&E) were carried out and levels of malondialdehyde (MDA) and glutathione (GSH) and activities of superoxide dismutase (SOD) in the liver were determined. After HFD feeding, the body weight gain and food efficiency ratio of HFD rats were significantly enhanced (p < 0.05vs. ND rats) and HAS treatment (18 and 36 mg/kg) significantly decreased the body weight gain and food efficiency ratio (p < 0.05vs. HFD rats). In addition, HAS treatment could decrease the abdominal adipose tissues and liver adipocytes. Furthermore, HAS treatment significantly decreased the T-CHO, TG, and LDL-C, whereas it increased HDL-C (p < 0.05vs. HFD rats) in serum and the liver. HAS treatment increased the GSH level and SOD activity in the liver (p < 0.05vs. HFD rats), whereas it decreased the levels of MDA (p < 0.05vs. HFD rats). mRNA analyses suggested that HAS treatment increases the expression of Pparg (proliferator-activated receptor γ) and Apoe (peroxisome apolipoprotein E). Immunohistochemistry and Western blotting indicated that HAS stimulation increased the levels of PPARγ and APOE in the liver, as a stress response of the body defense system. These results revealed that HAS exerts antiobesity and hypolipidemic activities in HFD rats by reducing liver oxidative stress and thus could be considered as a potential candidate drug to cure or prevent obesity and hyperlipidemia.
Conflict of interest statement
There are no conflicts of interest associated with this paper.
Figures






References
-
- Durkar A. M., Patil R. R., Naik S. R. Hypolipidemic and antioxidant activity of ethanolic extract of symplocos racemosa roxb. in hyperlipidemic rats: an evidence of participation of oxidative stress in hyperlipidemia. Indian Journal of Experimental Biology. 2014;52(1):36–45. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

