Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae
- PMID: 31534837
- PMCID: PMC6731771
- DOI: 10.7717/peerj.7489
Pyriproxyfen, a juvenile hormone analog, damages midgut cells and interferes with behaviors of Aedes aegypti larvae
Abstract
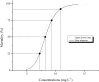
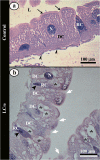
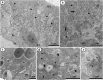
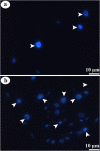
Juvenile hormone analogs (JHA) are known to interfere with growth and biosynthesis of insects with potential for insecticide action. However, there has been comparatively few data on morphological effects of JHA on insect organs. To determine pyriproxyfen effects on Aedes aegypti larvae, we conducted toxicity, behavioral bioassays and assessed ultrastructural effects of pyriproxyfen on midgut cells. A. aegypti larvae were exposed in aqueous solution of pyriproxyfen LC50 concentrations and evaluated for 24 h. This study fulfilled the toxic prevalence of pyriproxyfen to A. aegypti larvae (LC50 = 8.2 mg L-1). Behavioral observations confirmed that pyriproxyfen treatment significantly changes swimming behavior of larvae, limiting its displacement and speed. The pyriproxyfen causes remarkable histopathological and cytotoxic alterations in the midgut of larvae. Histopathological study reveals presence of cytoplasmic vacuolization and damage to brush border of the digestive cells. The main salient lesions of cytotoxic effects are occurrence of cell debris released into the midgut lumen, cytoplasm rich in lipid droplets, autophagosomes, disorganized microvilli and deformed mitochondria. Data suggest that pyriproxyfen can be used to help to control and eradicate this insect vector.
Keywords: Aedes aegypti; Autophagy; Juvenile Hormone; Locomotory behavior; Ultrastructure.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Behavioral and ultrastructural effects of novaluron on Aedes aegypti larvae.Infect Genet Evol. 2021 Sep;93:104974. doi: 10.1016/j.meegid.2021.104974. Epub 2021 Jun 21. Infect Genet Evol. 2021. PMID: 34166815
-
Midgut and fat body: Multisystemic action of pyriproxyfen on non-target organism Ceraeochrysa claveri (Neuroptera: Chrysopidae).Environ Pollut. 2022 Jan 15;293:118580. doi: 10.1016/j.envpol.2021.118580. Epub 2021 Nov 27. Environ Pollut. 2022. PMID: 34843850
-
Detrimental effects of pyriproxyfen on the detoxification and abilities of Belostoma anurum to prey upon Aedes aegypti larvae.Environ Pollut. 2021 Sep 1;284:117130. doi: 10.1016/j.envpol.2021.117130. Epub 2021 Apr 20. Environ Pollut. 2021. PMID: 33910136
-
Environmental risk assessment (ERA) of pyriproxyfen in non-target aquatic organisms.Aquat Toxicol. 2020 May;222:105448. doi: 10.1016/j.aquatox.2020.105448. Epub 2020 Feb 18. Aquat Toxicol. 2020. PMID: 32197184 Review.
-
Fate of Pyriproxyfen in Soils and Plants.Toxics. 2020 Mar 13;8(1):20. doi: 10.3390/toxics8010020. Toxics. 2020. PMID: 32183189 Free PMC article. Review.
Cited by
-
Evaluation of a mosquito home system for controlling Aedes aegypti.Parasit Vectors. 2021 Aug 18;14(1):413. doi: 10.1186/s13071-021-04918-9. Parasit Vectors. 2021. PMID: 34407881 Free PMC article.
-
Current Insights into Sublethal Effects of Pesticides on Insects.Int J Mol Sci. 2024 May 30;25(11):6007. doi: 10.3390/ijms25116007. Int J Mol Sci. 2024. PMID: 38892195 Free PMC article. Review.
-
Deltamethrin-Mediated Effects on Locomotion, Respiration, Feeding, and Histological Changes in the Midgut of Spodoptera frugiperda Caterpillars.Insects. 2021 May 22;12(6):483. doi: 10.3390/insects12060483. Insects. 2021. PMID: 34067273 Free PMC article.
-
Acute Toxicity and Sublethal Effects of Lemongrass Essential Oil and Their Components against the Granary Weevil, Sitophilus granarius.Insects. 2020 Jun 18;11(6):379. doi: 10.3390/insects11060379. Insects. 2020. PMID: 32570794 Free PMC article.
-
Side-effects of pesticides on non-target insects in agriculture: a mini-review.Naturwissenschaften. 2022 Feb 9;109(2):17. doi: 10.1007/s00114-022-01788-8. Naturwissenschaften. 2022. PMID: 35138481 Review.
References
-
- Aguiar RWS, Dos Santos SF, Da Silva Morgado F, Ascencio SD, De Mendonça Lopes M, Viana KF, Didonet J, Ribeiro BM. Insecticidal and repellent activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLOS ONE. 2015;10(2):e0116765. doi: 10.1371/journal.pone.0116765. - DOI - PMC - PubMed
-
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, Walter P. Molecular biology of the cell. Sixth Edition. New York: Garland Science; 2014.
-
- Alvarez Costa A, Gonzalez PV, Harburguer LV, Masuh HM. Effects of Temephos, Permethrin, and Eucalyptus nitens essential oil on survival and swimming behavior of Aedes aegypti and Anopheles pseudopunctipennis (Diptera: Culicidae) Larvae. Journal of Medical Entomology. 2018;55(5):1098–1104. doi: 10.1093/jme/tjy086. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources

