Type I-like behavior of the type II α7 nicotinic acetylcholine receptor positive allosteric modulator A-867744
- PMID: 31534841
- PMCID: PMC6727837
- DOI: 10.7717/peerj.7542
Type I-like behavior of the type II α7 nicotinic acetylcholine receptor positive allosteric modulator A-867744
Abstract
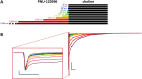
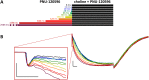
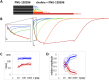
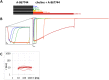
Cognitive impairment often involves the decreased expression or hypofunction of alpha 7-type nicotinic acetylcholine receptors (α7 nAChRs). Agonists or positive allosteric modulators (PAMs) of α7 nAChRs are known to be potential treatments for dementias, different neurodegenerative disorders, pain syndromes and conditions involving inflammation. In some of these conditions, it is desirable to maintain the temporal precision of fast cholinergic events, while in others, this temporal precision is unnecessary. For this reason, the optimal therapeutic effect for distinct indications may require PAMs with different mechanisms of action. The two major mechanisms are called "type I", which are compounds that augment α7 nAChR-mediated currents but maintain their characteristic fast kinetics; and "type II", which are compounds that produce augmented and prolonged currents. In this study, we performed a kinetic analysis of two type II PAMs of the α7 nAChR: PNU-120596 and A-867744, using a fast perfusion method that allowed high temporal resolution. We characterized the type of modulation produced by the two compounds, the state-dependence of the modulatory action, and the interaction between the two compounds. We found fundamental differences between the modulation mechanisms by PNU-120596 and A-867744. Most importantly, during brief agonist pulses, A-867744 caused a strikingly type I-like modulation, while PNU-120596 caused a type II-like prolonged activation. Our results demonstrate that specific compounds, even though all labeled as type II PAMs, can behave in completely different ways, including their onset and offset kinetics, state preference, and single channel open time. Our results emphasize that subtle details of the mechanism of action may be significant in assessing the therapeutic applicability of α7 nAChR PAM compounds.
Keywords: A-867744; Choline; Cognitive enhancement; PNU-120596; Patch clamp; Positive allosteric modulator; Type II PAM; α7 nAChR.
Conflict of interest statement
The authors declare there are no competing interests.
Figures








References
LinkOut - more resources
Full Text Sources
Miscellaneous

