Comment on "A commensal strain of Staphylococcus epidermidis protects against skin neoplasia" by Nakatsuji et al
- PMID: 31535021
- PMCID: PMC6739109
- DOI: 10.1126/sciadv.aaw3915
Comment on "A commensal strain of Staphylococcus epidermidis protects against skin neoplasia" by Nakatsuji et al
Abstract
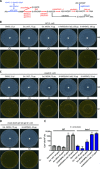
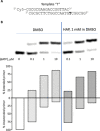
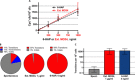
A recent article in Science Advances described the striking discovery that the commensal Staphylococcus epidermidis strain MO34 displays antimicrobial and antitumor activities by producing a small molecule, identified as the nucleobase analog 6-N-hydroxylaminopurine (6-HAP). However, in contradiction to the literature, the authors claimed that 6-HAP is nonmutagenic and proposed that the toxic effect of 6-HAP results from its ability to inhibit, in its base form, DNA synthesis. To resolve the discrepancy, we proved by genetic experiments with bacteria and yeast that extracts of MO34 do contain a mutagenic compound whose effects are identical to chemically synthesized 6-HAP. The MO34 extract induced the same mutation spectrum as authentic 6-HAP. Notably, the toxic and mutagenic effects of both synthetic and MO34-derived 6-HAP depended on conversion to the corresponding nucleotide. The nucleobase 6-HAP does not inhibit DNA synthesis in vitro, and we conclude that 6-HAP exerts its biological activity when incorporated into DNA.
Figures
Comment in
-
Response to Comment on "A commensal strain of Staphylococcus epidermidis protects against skin neoplasia" by Nakatsuji et al.Sci Adv. 2019 Sep 11;5(9):eaay5611. doi: 10.1126/sciadv.aay5611. eCollection 2019 Sep. Sci Adv. 2019. PMID: 31535030 Free PMC article.
Comment on
-
A commensal strain of Staphylococcus epidermidis protects against skin neoplasia.Sci Adv. 2018 Feb 28;4(2):eaao4502. doi: 10.1126/sciadv.aao4502. eCollection 2018 Feb. Sci Adv. 2018. PMID: 29507878 Free PMC article.
References
-
- Burke M. P., Borland K. M., Litosh V. A., Base-modified nucleosides as chemotherapeutic agents: past and future. Curr. Top. Med. Chem. 16, 1231–1241 (2016). - PubMed
-
- Kozmin S. G., Schaaper R. M., Shcherbakova P. V., Kulikov V. N., Noskov V. N., Guetsova M. L., Alenin V. V., Rogozin I. B., Makarova K. S., Pavlov Y. I., Multiple antimutagenesis mechanisms affect mutagenic activity and specificity of the base analog 6-N-hydroxylaminopurine in bacteria and yeast. Mutat. Res. 402, 41–50 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

