Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression
- PMID: 31535081
- PMCID: PMC6750232
- DOI: 10.1038/s42255-019-0039-6
Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression
Abstract
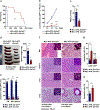
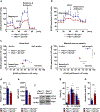
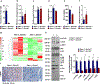
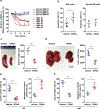
Amino acid (AA) metabolism is involved in diverse cellular functions, including cell survival and growth, however it remains unclear how it regulates normal hematopoiesis versus leukemogenesis. Here, we report that knockout of Slc1a5 (ASCT2), a transporter of neutral AAs, especially glutamine, results in mild to moderate defects in bone marrow and mature blood cell development under steady state conditions. In contrast, constitutive or induced deletion of Slc1a5 decreases leukemia initiation and maintenance driven by the oncogene MLL-AF9 or Pten deficiency. Survival of leukemic mice is prolonged following Slc1a5 deletion, and pharmacological inhibition of ASCT2 also decreases leukemia development and progression in xenograft models of human acute myeloid leukemia. Mechanistically, loss of ASCT2 generates a global effect on cellular metabolism, disrupts leucine influx and mTOR signaling, and induces apoptosis in leukemic cells. Given the substantial difference in reliance on ASCT2-mediated AA metabolism between normal and malignant blood cells, this in vivo study suggests ASCT2 as a promising therapeutic target for the treatment of leukemia.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

