Accelerated free-breathing whole-heart 3D T2 mapping with high isotropic resolution
- PMID: 31535729
- PMCID: PMC6899588
- DOI: 10.1002/mrm.27989
Accelerated free-breathing whole-heart 3D T2 mapping with high isotropic resolution
Abstract
Purpose: To enable free-breathing whole-heart 3D T2 mapping with high isotropic resolution in a clinically feasible and predictable scan time. This 3D motion-corrected undersampled signal matched (MUST) T2 map is achieved by combining an undersampled motion-compensated T2 -prepared Cartesian acquisition with a high-order patch-based reconstruction.
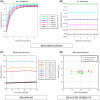
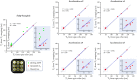
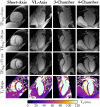
Methods: The 3D MUST-T2 mapping acquisition consists of an electrocardiogram-triggered, T2 -prepared, balanced SSFP sequence with nonselective saturation pulses. Three undersampled T2 -weighted volumes are acquired using a 3D Cartesian variable-density sampling with increasing T2 preparation times. A 2D image-based navigator is used to correct for respiratory motion of the heart and allow 100% scan efficiency. Multicontrast high-dimensionality undersampled patch-based reconstruction is used in concert with dictionary matching to generate 3D T2 maps. The proposed framework was evaluated in simulations, phantom experiments, and in vivo (10 healthy subjects, 2 patients) with 1.5-mm3 isotropic resolution. Three-dimensional MUST-T2 was compared against standard multi-echo spin-echo sequence (phantom) and conventional breath-held single-shot 2D SSFP T2 mapping (in vivo).
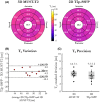
Results: Three-dimensional MUST-T2 showed high accuracy in phantom experiments (R2 > 0.99). The precision of T2 values was similar for 3D MUST-T2 and 2D balanced SSFP T2 mapping in vivo (5 ± 1 ms versus 4 ± 2 ms, P = .52). Slightly longer T2 values were observed with 3D MUST-T2 in comparison to 2D balanced SSFP T2 mapping (50.7 ± 2 ms versus 48.2 ± 1 ms, P < .05). Preliminary results in patients demonstrated T2 values in agreement with literature values.
Conclusion: The proposed approach enables free-breathing whole-heart 3D T2 mapping with high isotropic resolution in about 8 minutes, achieving accurate and precise T2 quantification of myocardial tissue in a clinically feasible scan time.
Keywords: T2 quantification; fast imaging; isotropic resolution; motion correction; myocardial T2 mapping.
© 2019 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Conflict of interest statement
Dr. Radhouene Neji is employed by Siemens Healthcare, Frimley, United Kingdom.
Figures



References
-
- He T, Gatehouse PD, Anderson LJ, et al. Development of a novel optimized breathhold technique for myocardial T2 measurement in thalassemia. J Magn Reson Imaging. 2006;24:580–585. - PubMed
-
- Haberkorn SM, Spieker M, Jacoby C, Flögel U, Kelm M, Bönner F. State of the art in cardiovascular T2 mapping: on the way to a cardiac biomarker? Curr Cardiovasc Imaging Rep. 2018;11.
-
- Van Heeswijk RB, Piccini D, Feliciano H, Hullin R, Schwitter J, Stuber M. Self‐navigated isotropic three‐dimensional cardiac T2 mapping. Magn Reson Med. 2015;73:1549–1554. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

