Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial
- PMID: 31536101
- PMCID: PMC6763993
- DOI: 10.1001/jama.2019.13772
Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial
Erratum in
-
Errors in Results Section.JAMA. 2019 Dec 3;322(21):2138. doi: 10.1001/jama.2019.19012. JAMA. 2019. PMID: 31794610 No abstract available.
Abstract
Importance: Type 2 diabetes is associated with increased cardiovascular risk. In placebo-controlled cardiovascular safety trials, the dipeptidyl peptidase-4 inhibitor linagliptin demonstrated noninferiority, but it has not been tested against an active comparator.
Objective: This trial assessed cardiovascular outcomes of linagliptin vs glimepiride (sulfonylurea) in patients with relatively early type 2 diabetes and risk factors for or established atherosclerotic cardiovascular disease.
Design, setting, and participants: Randomized, double-blind, active-controlled, noninferiority trial, with participant screening from November 2010 to December 2012, conducted at 607 hospital and primary care sites in 43 countries involving 6042 participants. Adults with type 2 diabetes, glycated hemoglobin of 6.5% to 8.5%, and elevated cardiovascular risk were eligible for inclusion. Elevated cardiovascular risk was defined as documented atherosclerotic cardiovascular disease, multiple cardiovascular risk factors, aged at least 70 years, and evidence of microvascular complications. Follow-up ended in August 2018.
Interventions: Patients were randomized to receive 5 mg of linagliptin once daily (n = 3023) or 1 to 4 mg of glimepiride once daily (n = 3010) in addition to usual care. Investigators were encouraged to intensify glycemic treatment, primarily by adding or adjusting metformin, α-glucosidase inhibitors, thiazolidinediones, or insulin, according to clinical need.
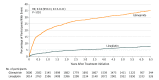
Main outcomes and measures: The primary outcome was time to first occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke with the aim to establish noninferiority of linagliptin vs glimepiride, defined by the upper limit of the 2-sided 95.47% CI for the hazard ratio (HR) of linagliptin relative to glimepiride of less than 1.3.
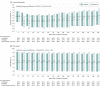
Results: Of 6042 participants randomized, 6033 (mean age, 64.0 years; 2414 [39.9%] women; mean glycated hemoglobin, 7.2%; median duration of diabetes, 6.3 years; 42% with macrovascular disease; 59% had undergone metformin monotherapy) were treated and analyzed. The median duration of follow-up was 6.3 years. The primary outcome occurred in 356 of 3023 participants (11.8%) in the linagliptin group and 362 of 3010 (12.0%) in the glimepiride group (HR, 0.98 [95.47% CI, 0.84-1.14]; P < .001 for noninferiority), meeting the noninferiority criterion but not superiority (P = .76). Adverse events occurred in 2822 participants (93.4%) in the linagliptin group and 2856 (94.9%) in the glimepiride group, with 15 participants (0.5%) in the linagliptin group vs 16 (0.5%) in the glimepiride group with adjudicated-confirmed acute pancreatitis. At least 1 episode of hypoglycemic adverse events occurred in 320 (10.6%) participants in the linagliptin group and 1132 (37.7%) in the glimepiride group (HR, 0.23 [95% CI, 0.21-0.26]).
Conclusions and relevance: Among adults with relatively early type 2 diabetes and elevated cardiovascular risk, the use of linagliptin compared with glimepiride over a median 6.3 years resulted in a noninferior risk of a composite cardiovascular outcome.
Trial registration: ClinicalTrials.gov Identifier: NCT01243424.
Conflict of interest statement
Figures


References
-
- Davies MJ, D’Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018: a consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. doi:10.2337/dci18-0033 - DOI - PMC - PubMed
-
- Das SR, Everett BM, Birtcher KK, et al. . 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200-3223. doi:10.1016/j.jacc.2018.09.020 - DOI - PMC - PubMed
-
- Guidelines on Second- and Third-Line Medicines and Type of Insulin for the Control of Blood Glucose Levels in Non-Pregnant Adults With Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 2018 . http://apps.who.int/iris/bitstream/handle/10665/272433/9789241550284-eng.... - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

