In utero Bisphenol A Exposure Is Linked with Sex Specific Changes in the Transcriptome and Methylome of Human Amniocytes
- PMID: 31536135
- PMCID: PMC7046022
- DOI: 10.1210/clinem/dgz037
In utero Bisphenol A Exposure Is Linked with Sex Specific Changes in the Transcriptome and Methylome of Human Amniocytes
Abstract
Context: Prenatal exposure to bisphenol A (BPA) is linked to obesity and diabetes but the molecular mechanisms driving these phenomena are not known. Alterations in deoxyribonucleic acid (DNA) methylation in amniocytes exposed to BPA in utero represent a potential mechanism leading to metabolic dysfunction later in life.
Objective: To profile changes in genome-wide DNA methylation and expression in second trimester human amniocytes exposed to BPA in utero.
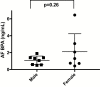
Design: A nested case-control study was performed in amniocytes matched for offspring sex, maternal race/ethnicity, maternal age, gestational age at amniocentesis, and gestational age at birth. Cases had amniotic fluid BPA measuring 0.251 to 23.74 ng/mL. Sex-specific genome-wide DNA methylation analysis and RNA-sequencing (RNA-seq) were performed to determine differentially methylated regions (DMRs) and gene expression changes associated with BPA exposure. Ingenuity pathway analysis was performed to identify biologically relevant pathways enriched after BPA exposure. In silico Hi-C analysis identified potential chromatin interactions with DMRs.
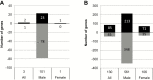
Results: There were 101 genes with altered expression in male amniocytes exposed to BPA (q < 0.05) in utero, with enrichment of pathways critical to hepatic dysfunction, collagen signaling and adipogenesis. Thirty-six DMRs were identified in male BPA-exposed amniocytes and 14 in female amniocyte analysis (q < 0.05). Hi-C analysis identified interactions between DMRs and 24 genes with expression changes in male amniocytes and 12 in female amniocytes (P < 0.05).
Conclusion: In a unique repository of human amniocytes exposed to BPA in utero, sex-specific analyses identified gene expression changes in pathways associated with metabolic disease and novel DMRs with potential distal regulatory functions.
© Endocrine Society 2019. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




Similar articles
-
Exposure to Gestational Diabetes Enriches Immune-Related Pathways in the Transcriptome and Methylome of Human Amniocytes.J Clin Endocrinol Metab. 2020 Oct 1;105(10):3250-64. doi: 10.1210/clinem/dgaa466. J Clin Endocrinol Metab. 2020. PMID: 32687192 Free PMC article.
-
Sex- and Dose-Specific Effects of Maternal Bisphenol A Exposure on Pancreatic Islets of First- and Second-Generation Adult Mice Offspring.Environ Health Perspect. 2017 Sep 27;125(9):097022. doi: 10.1289/EHP1674. Environ Health Perspect. 2017. PMID: 29161229 Free PMC article.
-
An epigenome-wide analysis of cord blood DNA methylation reveals sex-specific effect of exposure to bisphenol A.Sci Rep. 2019 Aug 26;9(1):12369. doi: 10.1038/s41598-019-48916-5. Sci Rep. 2019. PMID: 31451752 Free PMC article.
-
Association of BPA exposure during pregnancy with risk of preterm birth and changes in gestational age: A meta-analysis and systematic review.Ecotoxicol Environ Saf. 2021 Sep 1;220:112400. doi: 10.1016/j.ecoenv.2021.112400. Epub 2021 Jun 8. Ecotoxicol Environ Saf. 2021. PMID: 34116331
-
Epigenetic perspective on the developmental effects of bisphenol A.Brain Behav Immun. 2011 Aug;25(6):1084-93. doi: 10.1016/j.bbi.2011.02.005. Epub 2011 Feb 17. Brain Behav Immun. 2011. PMID: 21333735 Free PMC article. Review.
Cited by
-
Comparative in silico and in vitro evaluation of possible toxic effects of bisphenol derivatives in HepG2 cells.Toxicol Res (Camb). 2024 Aug 11;13(4):tfae127. doi: 10.1093/toxres/tfae127. eCollection 2024 Aug. Toxicol Res (Camb). 2024. PMID: 39132192 Free PMC article.
-
Sex-specific effects of bisphenol A on the signaling pathway of ESRRG in the human placenta†.Biol Reprod. 2022 Jun 13;106(6):1278-1291. doi: 10.1093/biolre/ioac044. Biol Reprod. 2022. PMID: 35220427 Free PMC article.
-
Environmental Exposure to Endocrine Disrupting Chemicals Influences Genomic Imprinting, Growth, and Metabolism.Genes (Basel). 2021 Jul 28;12(8):1153. doi: 10.3390/genes12081153. Genes (Basel). 2021. PMID: 34440327 Free PMC article. Review.
-
Identification of Novel Regulatory Regions Induced by Intrauterine Growth Restriction in Rat Islets.Endocrinology. 2022 Feb 1;163(2):bqab251. doi: 10.1210/endocr/bqab251. Endocrinology. 2022. PMID: 34894232 Free PMC article.
-
Distinct epigenetic modulation of differentially expressed genes in the adult mouse brain following prenatal exposure to low-dose bisphenol A.Cell Biol Toxicol. 2024 May 22;40(1):37. doi: 10.1007/s10565-024-09875-4. Cell Biol Toxicol. 2024. PMID: 38777957 Free PMC article.
References
-
- Heindel JJ, Skalla LA, Joubert BR, Dilworth CH, Gray KA. Review of developmental origins of health and disease publications in environmental epidemiology. Reprod Toxicol. 2017;68:34–48. - PubMed
-
- Shafei AE, Nabih ES, Shehata KA, et al. Prenatal exposure to endocrine disruptors and reprogramming of adipogenesis: an early-life risk factor for childhood obesity. Child Obes. 2018;14(1):18–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

