Genotype correlates with clinical severity in PIK3CA-associated lymphatic malformations
- PMID: 31536475
- PMCID: PMC6948764
- DOI: 10.1172/jci.insight.129884
Genotype correlates with clinical severity in PIK3CA-associated lymphatic malformations
Abstract
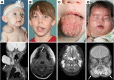
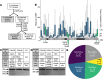
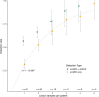
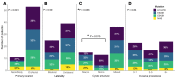
Lymphatic malformations (LMs) are congenital, nonneoplastic vascular malformations associated with postzygotic activating PIK3CA mutations. The mutation spectrum within LMs is narrow, with the majority having 1 of 3 hotspot mutations. Despite this relative genetic homogeneity, clinical presentations differ dramatically. We used molecular inversion probes and droplet digital polymerase chain reaction to perform deep, targeted sequencing of PIK3CA in 271 affected and unaffected tissue samples from 81 individuals with isolated LMs and retrospectively collected clinical data. Pathogenic PIK3CA mutations were identified in affected LM tissue in 64 individuals (79%) with isolated LMs, with variant allele fractions (VAFs) ranging from 0.1% to 13%. Initial analyses revealed no correlation between VAF and phenotype variables. Recognizing that different mutations activate PI3K to varying degrees, we developed a metric, the genotype-adjusted VAF (GVAF), to account for differences in mutation strength, and found significantly higher GVAFs in LMs with more severe clinical characteristics including orofacial location or microcystic structure. In addition to providing insight into LM pathogenesis, we believe GVAF may have broad applicability for genotype-phenotype analyses in mosaic disorders.
Keywords: Genetics; Molecular genetics; Vascular Biology.
Conflict of interest statement
Figures

References
-
- Flint PW, et al. Cummings Otolaryngology, 6th Edition. Amsterdam, The Netherlands: Elsevier; 2015. https://www.elsevier.com/books/cummings-otolaryngology/flint/978-1-4557-....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

