Exenatide regulates pancreatic islet integrity and insulin sensitivity in the nonhuman primate baboon Papio hamadryas
- PMID: 31536476
- PMCID: PMC6824445
- DOI: 10.1172/jci.insight.93091
Exenatide regulates pancreatic islet integrity and insulin sensitivity in the nonhuman primate baboon Papio hamadryas
Abstract
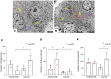
The glucagon-like peptide-1 receptor agonist exenatide improves glycemic control by several and not completely understood mechanisms. Herein, we examined the effects of chronic intravenous exenatide infusion on insulin sensitivity, β cell and α cell function and relative volumes, and islet cell apoptosis and replication in nondiabetic nonhuman primates (baboons). At baseline, baboons received a 2-step hyperglycemic clamp followed by an l-arginine bolus (HC/A). After HC/A, baboons underwent a partial pancreatectomy (tail removal) and received a continuous exenatide (n = 12) or saline (n = 12) infusion for 13 weeks. At the end of treatment, HC/A was repeated, and the remnant pancreas (head-body) was harvested. Insulin sensitivity increased dramatically after exenatide treatment and was accompanied by a decrease in insulin and C-peptide secretion, while the insulin secretion/insulin resistance (disposition) index increased by about 2-fold. β, α, and δ cell relative volumes in exenatide-treated baboons were significantly increased compared with saline-treated controls, primarily as the result of increased islet cell replication. Features of cellular stress and secretory dysfunction were present in islets of saline-treated baboons and absent in islets of exenatide-treated baboons. In conclusion, chronic administration of exenatide exerts proliferative and cytoprotective effects on β, α, and δ cells and produces a robust increase in insulin sensitivity in nonhuman primates.
Keywords: B cells; Endocrinology; Glucose metabolism; Insulin signaling.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

