Interim report on the effective intraperitoneal therapy of insulin-dependent diabetes mellitus in pet dogs using "Neo-Islets," aggregates of adipose stem and pancreatic islet cells (INAD 012-776)
- PMID: 31536503
- PMCID: PMC6752848
- DOI: 10.1371/journal.pone.0218688
Interim report on the effective intraperitoneal therapy of insulin-dependent diabetes mellitus in pet dogs using "Neo-Islets," aggregates of adipose stem and pancreatic islet cells (INAD 012-776)
Abstract
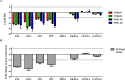
We previously reported that allogeneic, intraperitoneally administered "Neo-Islets," composed of cultured pancreatic islet cells co-aggregated with high numbers of immunoprotective and cytoprotective Adipose-derived Stem Cells, reestablished, through omental engraftment, redifferentiation and splenic and omental up-regulation of regulatory T-cells, normoglycemia in autoimmune Type-1 Diabetic Non-Obese Diabetic (NOD) mice without the use of immunosuppressive agents or encapsulation devices. Based on these observations, we are currently testing this Neo-Islet technology in an FDA guided pilot study (INAD 012-776) in insulin-dependent, spontaneously diabetic pet dogs by ultrasound-guided, intraperitoneal administration of 2x10e5 Neo-Islets/kilogram body weight to metabolically controlled (blood glucose, triglycerides, thyroid and adrenal functions) and sedated animals. We report here interim observations on the first 4 canine Neo-Islet-treated, insulin-dependent pet dogs that are now in the early to intermediate-term follow-up phase of the planned 3 year study (> 6 months post treatment). Current results from this translational study indicate that in dogs, Neo-Islets appear to engraft, redifferentiate and physiologically produce insulin, and are rejected by neither auto- nor allo-immune responses, as evidenced by (a) an absent IgG response to the allogeneic cells contained in the administered Neo-Islets, and (b) progressively improved glycemic control that achieves up to a 50% reduction in daily insulin needs paralleled by a statistically significant decrease in serum glucose concentrations. This is accomplished without the use of anti-rejection drugs or encapsulation devices. No adverse or serious adverse events related to the Neo-Islet administration have been observed to date. We conclude that this minimally invasive therapy has significant translational relevance to veterinary and clinical Type 1 diabetes mellitus by achieving complete and at this point partial glycemic control in two species, i.e., diabetic mice and dogs, respectively.
Conflict of interest statement
The following authors, AG, PZ, ZH are employees of SymbioCellTech, LLC; and CW is a consultant to SymbioCellTech, LLC. CW, AG, PZ, ZH are shareholders in SymbioCellTech, LLC, and declare competing financial interests. A patent is pending on the herein described technology. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


Similar articles
-
Significant expansion of the donor pool achieved by utilizing islets of variable quality in the production of allogeneic "Neo-Islets", 3-D organoids of Mesenchymal Stromal and islet cells, a novel immune-isolating biotherapy for Type I Diabetes.PLoS One. 2023 Aug 24;18(8):e0290460. doi: 10.1371/journal.pone.0290460. eCollection 2023. PLoS One. 2023. PMID: 37616230 Free PMC article.
-
Intraperitoneal administration of human "Neo-Islets", 3-D organoids of mesenchymal stromal and pancreatic islet cells, normalizes blood glucose levels in streptozotocin-diabetic NOD/SCID mice: Significance for clinical trials.PLoS One. 2021 Oct 28;16(10):e0259043. doi: 10.1371/journal.pone.0259043. eCollection 2021. PLoS One. 2021. PMID: 34710142 Free PMC article.
-
Durable Control of Autoimmune Diabetes in Mice Achieved by Intraperitoneal Transplantation of "Neo-Islets," Three-Dimensional Aggregates of Allogeneic Islet and "Mesenchymal Stem Cells".Stem Cells Transl Med. 2017 Jul;6(7):1631-1643. doi: 10.1002/sctm.17-0005. Epub 2017 May 3. Stem Cells Transl Med. 2017. PMID: 28467694 Free PMC article.
-
Transplantation of pancreatic islets contained in minimal volume microcapsules in diabetic high mammalians.Ann N Y Acad Sci. 1999 Jun 18;875:219-32. doi: 10.1111/j.1749-6632.1999.tb08506.x. Ann N Y Acad Sci. 1999. PMID: 10415570 Review.
-
Pancreatic islet transplantation: from dogs to humans and back again.Vet Surg. 2014 Aug;43(6):631-41. doi: 10.1111/j.1532-950X.2014.12224.x. Epub 2014 Jun 9. Vet Surg. 2014. PMID: 24909456 Review.
Cited by
-
Stem Cells in Veterinary Medicine-Current State and Treatment Options.Front Vet Sci. 2020 May 29;7:278. doi: 10.3389/fvets.2020.00278. eCollection 2020. Front Vet Sci. 2020. PMID: 32656249 Free PMC article. Review.
-
Strategy for Clinical Setting of Co-transplantation of Mesenchymal Stem Cells and Pancreatic Islets.Cell Transplant. 2024 Jan-Dec;33:9636897241259433. doi: 10.1177/09636897241259433. Cell Transplant. 2024. PMID: 38877672 Free PMC article. Review.
-
Feline Adipose Derived Multipotent Stromal Cell Transdifferentiation Into Functional Insulin Producing Cell Clusters.Front Bioeng Biotechnol. 2022 Jun 8;10:904519. doi: 10.3389/fbioe.2022.904519. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35769100 Free PMC article.
-
Significant expansion of the donor pool achieved by utilizing islets of variable quality in the production of allogeneic "Neo-Islets", 3-D organoids of Mesenchymal Stromal and islet cells, a novel immune-isolating biotherapy for Type I Diabetes.PLoS One. 2023 Aug 24;18(8):e0290460. doi: 10.1371/journal.pone.0290460. eCollection 2023. PLoS One. 2023. PMID: 37616230 Free PMC article.
-
Intraperitoneal administration of human "Neo-Islets", 3-D organoids of mesenchymal stromal and pancreatic islet cells, normalizes blood glucose levels in streptozotocin-diabetic NOD/SCID mice: Significance for clinical trials.PLoS One. 2021 Oct 28;16(10):e0259043. doi: 10.1371/journal.pone.0259043. eCollection 2021. PLoS One. 2021. PMID: 34710142 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

