Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the National Cancer Database
- PMID: 31536530
- PMCID: PMC6752843
- DOI: 10.1371/journal.pone.0222358
Clinical outcomes with neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer: A report from the National Cancer Database
Abstract
Purpose: Triple negative breast cancer (TNBC) patients frequently receive neoadjuvant chemotherapy (NAC). Only 50% will achieve pathological complete response (pCR). In this retrospective study, we evaluated TNBC outcomes with NAC vs. AC.
Methods: Patients with stages II and III TNBC treated with NAC or AC between 2010 and 2013 were identified from the National Cancer Database. Baseline characteristics were compared with χ2 and two sample t tests. Kaplan-Meier survival analyses were computed in patients treated with NAC or AC, and log-rank tests used to examine differences. Unadjusted analyses of trends in proportions over time were performed using Cochran-Armitage tests. Log-binomial models were applied to estimate relative risks of non-pCR following NAC.
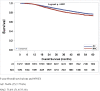
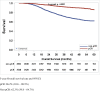
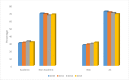
Results: Of 19,151 patients, 5,621 (29.4%) received NAC, 13,530 (70.6%) received AC. NAC treated patients had worse OS compared to AC treated patients (73.4% vs. 76.8%; p<0.0001). pCR rate following NAC was 47.4%, and was associated with improved 5 year OS compared to non-pCR (86.2% vs. 62.3%; p<0.0001). In patients who received NAC, age, black race, clinical stage, diagnosis year, and Charlson-Deyo comorbidity score predicted non-pCR status. Use of NAC increased over the study period from 2010 to 2013 (27.8% - 31.2%; p = 0.0002).
Conclusions: NAC may be inferior to AC in TNBC, likely related to the high frequency of non-pCR following NAC. It is unclear if removing the primary tumor prior to chemotherapy will have a beneficial biologic impact on therapeutic efficacy. These data should be considered hypothesis-generating as it is possible that the findings are due to selection bias, as physicians may use NAC for TNBC patients with more advanced local disease. Although, NAC still has a role in TNBC, developing biomarkers to identify patients likely to achieve pCR and benefit from NAC is an urgent need.
Conflict of interest statement
Foluso Ademuyiwa has served as an advisory board member for Eisai, Astra Zeneca, Jounce, Immunomedics, and QED Therapeutics. Foluso Ademuyiwa performs consulting for Cardinal Health, Best Doctors, Advance Medical Inc. Foluso Ademuyiwa has received institutional research funding from Polyphor, Pfizer, RNA Diagnostics, Immunomedics, Abbvie, and Seattle Genetics. Nusayba Bagegni declares that she has no conflict of interest. Yu Tao declares that she has no conflict of interest. Foluso Ademuyiwa has served as an advisory board member for Eisai, Astra Zeneca, Jounce, Immunomedics, and QED Therapeutics. Foluso Ademuyiwa performs consulting for Cardinal Health, Best Doctors, Advance Medical Inc. Foluso Ademuyiwa has received institutional research funding from Polyphor, Pfizer, RNA Diagnostics, Immunomedics, Abbvie, and Seattle Genetics. Nusayba Bagegni declares that she has no conflict of interest. Yu Tao declares that she has no conflict of interest. This does not alter our adherence to PLOS ONE policies on sharing data and materials
Figures

References
-
- Surveillance E, and End Results (SEER). Cancer Stat Facts: Female Breast Cancer 2018. [cited 2018 September 13, 2018].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

