Proton pump inhibitors attenuate myofibroblast formation associated with thyroid eye disease through the aryl hydrocarbon receptor
- PMID: 31536596
- PMCID: PMC6752849
- DOI: 10.1371/journal.pone.0222779
Proton pump inhibitors attenuate myofibroblast formation associated with thyroid eye disease through the aryl hydrocarbon receptor
Abstract
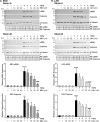
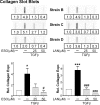
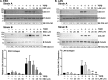
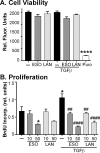
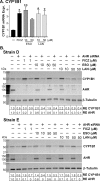
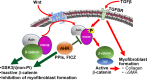
Thyroid eye disease (TED) can lead to scar formation and tissue remodeling in the orbital space. In severe cases, the scarring process leads to sight-threatening pathophysiology. There is no known effective way to prevent scar formation in TED patients, or to reverse scarring once it occurs. In this study, we show that the proton pump inhibitors (PPIs), esomeprazole and lansoprazole, can prevent transforming growth factor beta (TGFβ)-mediated differentiation of TED orbital fibroblasts to myofibroblasts, a critical step in scar formation. Both PPIs prevent TGFβ-induced increases in alpha-smooth muscle actin (αSMA), calponin, and collagen production and reduce TED orbital fibroblast cell proliferation and migration. Esomeprazole and lansoprazole exert these effects through an aryl hydrocarbon receptor (AHR)-dependent pathway that includes reducing β-catenin/Wnt signaling. We conclude that PPIs are potentially useful therapies for preventing or treating TED by reducing the myofibroblast accumulation that occurs in the disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The aryl hydrocarbon receptor pathway controls matrix metalloproteinase-1 and collagen levels in human orbital fibroblasts.Sci Rep. 2020 May 21;10(1):8477. doi: 10.1038/s41598-020-65414-1. Sci Rep. 2020. PMID: 32439897 Free PMC article.
-
The Aryl Hydrocarbon Receptor and Its Ligands Inhibit Myofibroblast Formation and Activation: Implications for Thyroid Eye Disease.Am J Pathol. 2016 Dec;186(12):3189-3202. doi: 10.1016/j.ajpath.2016.08.017. Epub 2016 Nov 11. Am J Pathol. 2016. PMID: 27842700 Free PMC article.
-
Tapinarof, an Aryl Hydrocarbon Receptor Ligand, Mitigates Fibroblast Activation in Thyroid Eye Disease: Implications for Novel Therapy.Invest Ophthalmol Vis Sci. 2024 Nov 4;65(13):40. doi: 10.1167/iovs.65.13.40. Invest Ophthalmol Vis Sci. 2024. PMID: 39560627 Free PMC article.
-
Molecular biomarkers of Graves' ophthalmopathy.Exp Mol Pathol. 2019 Feb;106:1-6. doi: 10.1016/j.yexmp.2018.11.004. Epub 2018 Nov 8. Exp Mol Pathol. 2019. PMID: 30414981 Free PMC article. Review.
-
Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem.Int J Clin Pharmacol Ther. 2006 Jul;44(7):297-302. doi: 10.5414/cpp44297. Int J Clin Pharmacol Ther. 2006. PMID: 16961157 Review.
Cited by
-
MicroRNA-130a Is Elevated in Thyroid Eye Disease and Increases Lipid Accumulation in Fibroblasts Through the Suppression of AMPK.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):29. doi: 10.1167/iovs.62.1.29. Invest Ophthalmol Vis Sci. 2021. PMID: 33507228 Free PMC article.
-
Is it possible to use Proton Pump Inhibitors in COVID-19 treatment and prophylaxis?Med Hypotheses. 2020 Oct;143:110018. doi: 10.1016/j.mehy.2020.110018. Epub 2020 Jun 20. Med Hypotheses. 2020. PMID: 32679422 Free PMC article.
-
The aryl hydrocarbon receptor pathway controls matrix metalloproteinase-1 and collagen levels in human orbital fibroblasts.Sci Rep. 2020 May 21;10(1):8477. doi: 10.1038/s41598-020-65414-1. Sci Rep. 2020. PMID: 32439897 Free PMC article.
-
AhR Mediated Activation of Pro-Inflammatory Response of RAW 264.7 Cells Modulate the Epithelial-Mesenchymal Transition.Toxics. 2022 Oct 27;10(11):642. doi: 10.3390/toxics10110642. Toxics. 2022. PMID: 36355934 Free PMC article.
-
Ceruloplasmin regulating fibrosis in orbital fibroblasts provides a novel therapeutic target for Graves' orbitopathy.J Endocrinol Invest. 2023 Oct;46(10):2005-2016. doi: 10.1007/s40618-023-02033-3. Epub 2023 Feb 27. J Endocrinol Invest. 2023. PMID: 36849849
References
-
- Schluter A, Horstmann M, Diaz-Cano S, Plohn S, Stahr K, Mattheis S, et al. Genetic immunization with mouse thyrotrophin hormone receptor plasmid breaks self-tolerance for a murine model of autoimmune thyroid disease and Graves' orbitopathy. Clin Exp Immunol. 2018;191(3):255–67. Epub 2017/10/24. 10.1111/cei.13075 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

