The amygdala differentially regulates defensive behaviors evoked by CO2
- PMID: 31536735
- PMCID: PMC6829583
- DOI: 10.1016/j.bbr.2019.112236
The amygdala differentially regulates defensive behaviors evoked by CO2
Abstract
CO2 inhalation can provoke panic attacks in humans, and the likelihood is increased in patients with panic disorder. Identifying brain sites involved could provide important mechanistic insight into the illness. In mice, the amygdala has been suggested to promote CO2 responses; however, recent studies in humans with amygdala damage indicate the amygdala is not required for CO2-induced fear and panic and might actually oppose these responses. To clarify the role of the amygdala, we produced lesions in mice paralleling the human lesions, and characterized behavioral responses to CO2. Compared to sham controls, we found that amygdala-lesioned mice froze less to 10% CO2, and unlike shams they also began to jump frenetically. At 20% CO2, controls also exhibited jumping, suggesting it is a normal response to more extreme CO2 concentrations. The effect of amygdala lesions was specific to CO2 as amygdala-lesioned mice did not jump in response to a predator odor or to an auditory conditioned stimulus. In amygdala-lesioned mice, jumping evoked by 10% CO2 was eliminated by co-lesioning the dorsal periaqueductal gray, a structure implicated in panic and escape-related behaviors. Together, these observations suggest a dual role for the amygdala in the CO2 response: promoting CO2-induced freezing, and opposing CO2-induced jumping, which may help explain the exaggerated CO2 responses in humans with amygdala lesions.
Keywords: Amygdala; Carbon dioxide; Panic; Periacqueductal gray.
Published by Elsevier B.V.
Figures
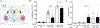
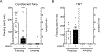

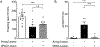
Similar articles
-
Evidence of a suffocation alarm system within the periaqueductal gray matter of the rat.Neuroscience. 2012 Jan 3;200:59-73. doi: 10.1016/j.neuroscience.2011.10.032. Epub 2011 Oct 25. Neuroscience. 2012. PMID: 22062132
-
Short-term full kindling of the amygdala dissociates natural and periaqueductal gray-evoked flight behaviors of the rat.Behav Brain Res. 2009 May 16;199(2):247-56. doi: 10.1016/j.bbr.2008.11.042. Epub 2008 Dec 3. Behav Brain Res. 2009. PMID: 19103230
-
The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis.J Neurosci. 2014 Jul 30;34(31):10247-55. doi: 10.1523/JNEUROSCI.1680-14.2014. J Neurosci. 2014. PMID: 25080586 Free PMC article.
-
Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety.Behav Brain Res. 2008 Mar 17;188(1):1-13. doi: 10.1016/j.bbr.2007.10.018. Epub 2007 Oct 25. Behav Brain Res. 2008. PMID: 18054397 Review.
-
Serotonin, the periaqueductal gray and panic.Neurosci Biobehav Rev. 2004 May;28(3):239-59. doi: 10.1016/j.neubiorev.2003.12.004. Neurosci Biobehav Rev. 2004. PMID: 15225969 Review.
Cited by
-
Brain Mechanisms Underlying Panic Attack and Panic Disorder.Neurosci Bull. 2024 Jun;40(6):795-814. doi: 10.1007/s12264-023-01088-9. Epub 2023 Jul 21. Neurosci Bull. 2024. PMID: 37477800 Free PMC article. Review.
-
Amygdala-driven apnea and the chemoreceptive origin of anxiety.Biol Psychol. 2022 Apr;170:108305. doi: 10.1016/j.biopsycho.2022.108305. Epub 2022 Mar 7. Biol Psychol. 2022. PMID: 35271957 Free PMC article. Review.
-
A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy.JCI Insight. 2020 Mar 26;5(6):e134852. doi: 10.1172/jci.insight.134852. JCI Insight. 2020. PMID: 32163374 Free PMC article.
-
Estrogens, age, and, neonatal stress: panic disorders and novel views on the contribution of non-medullary structures to respiratory control and CO2 responses.Front Physiol. 2023 May 17;14:1183933. doi: 10.3389/fphys.2023.1183933. eCollection 2023. Front Physiol. 2023. PMID: 37265841 Free PMC article. Review.
-
Neuromedin B-Expressing Neurons in the Retrotrapezoid Nucleus Regulate Respiratory Homeostasis and Promote Stable Breathing in Adult Mice.J Neurosci. 2023 Jul 26;43(30):5501-5520. doi: 10.1523/JNEUROSCI.0386-23.2023. Epub 2023 Jun 8. J Neurosci. 2023. PMID: 37290937 Free PMC article.
References
-
- Blanchard DC, Griebel G, Pobbe R, Blanchard RJ, Risk assessment as an evolved threat detection and analysis process, Neurosci Biobehav Rev 35(4) (2011) 991–8. - PubMed
-
- Eilam D, Die hard: a blend of freezing and fleeing as a dynamic defense--implications for the control of defensive behavior, Neurosci Biobehav Rev 29(8) (2005) 1181–91. - PubMed
-
- Dielenberg RA, McGregor IS, Defensive behavior in rats towards predatory odors: a review, Neurosci Biobehav Rev 25(7–8) (2001) 597–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

