From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week
- PMID: 31537270
- PMCID: PMC6910128
- DOI: 10.1016/j.jacc.2019.07.061
From Focal Lipid Storage to Systemic Inflammation: JACC Review Topic of the Week
Abstract
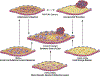
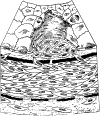
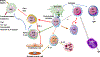
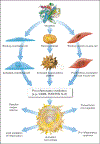
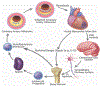
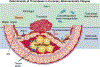
Concepts of atherogenesis have evolved considerably with time. Early animal experiments showed that a cholesterol-rich diet could induce fatty lesion formation in arteries. The elucidation of lipoprotein metabolism ultimately led to demonstrating the clinical benefits of lipid lowering. The view of atheromata as bland accumulations of smooth muscle cells that elaborated an extracellular matrix that could entrap lipids then expanded to embrace inflammation as providing pathways that could link risk factors to atherogenesis. The characterization of leukocyte adhesion molecules and their control by proinflammatory cytokines, the ability of chemokines to recruit leukocytes, and the identification of inflammatory cell subtypes in lesions spurred the unraveling of innate and adaptive immune pathways that contribute to atherosclerosis and its thrombotic complications. Such pathophysiologic insights have led to the identification of biomarkers that can define categories of risk and direct therapies and to the development of new treatments.
Keywords: LDL cholesterol; inflammation; smooth muscle cell.
Copyright © 2019 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Virchow R Cellular Pathology. London: John Churchill, 1858.
-
- Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present--on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch 2006;449:96–103. - PubMed
-
- Duguid J Thrombosis as a factor in the pathogenesis of coronary atherosclerosis. J Pathol 1946;58:208–212. - PubMed
-
- Anitchkov N, Chalatow S. On experimental cholesterin steatosis and its significance in the origin of some pathological processes (1913). Reprinted in Arteriosclerosis 1983;3:178–182. - PubMed
-
- Anitchkov N Über die Veränderungen der Kaninchenaorta bei experimenteller Cholesterin-steatose. Beitr Pathol Anat 1913;56:379–404.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

