Co-trimoxazole or multivitamin multimineral supplement for post-discharge outcomes after severe anaemia in African children: a randomised controlled trial
- PMID: 31537373
- PMCID: PMC7024999
- DOI: 10.1016/S2214-109X(19)30345-6
Co-trimoxazole or multivitamin multimineral supplement for post-discharge outcomes after severe anaemia in African children: a randomised controlled trial
Abstract
Background: Severe anaemia is a leading cause of paediatric admission to hospital in Africa; post-discharge outcomes remain poor, with high 6-month mortality (8%) and re-admission (17%). We aimed to investigate post-discharge interventions that might improve outcomes.
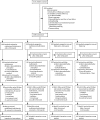
Methods: Within the two-stratum, open-label, multicentre, factorial randomised TRACT trial, children aged 2 months to 12 years with severe anaemia, defined as haemoglobin of less than 6 g/dL, at admission to hospital (three in Uganda, one in Malawi) were randomly assigned, using sequentially numbered envelopes linked to a second non-sequentially numbered set of allocations stratified by centre and severity, to enhanced nutritional supplementation with iron and folate-containing multivitamin multimineral supplements versus iron and folate alone at treatment doses (usual care), and to co-trimoxazole versus no co-trimoxazole. All interventions were administered orally and were given for 3 months after discharge from hospital. Separately reported randomisations investigated transfusion management. The primary outcome was 180-day mortality. All analyses were done in the intention-to-treat population; follow-up was 180 days. This trial is registered with the International Standard Randomised Controlled Trial registry, ISRCTN84086586, and follow-up is complete.
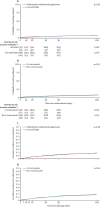
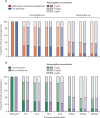
Findings: From Sept 17, 2014, to May 15, 2017, 3983 eligible children were randomly assigned to treatment, and followed up for 180 days. 164 (4%) were lost to follow-up. 1901 (95%) of 1997 assigned multivitamin multimineral supplement, 1911 (96%) of 1986 assigned iron and folate, and 1922 (96%) of 1994 assigned co-trimoxazole started treatment. By day 180, 166 (8%) children in the multivitamin multimineral supplement group versus 169 (9%) children in the iron and folate group had died (hazard ratio [HR] 0·97, 95% CI 0·79-1·21; p=0·81) and 172 (9%) who received co-trimoxazole versus 163 (8%) who did not receive co-trimoxazole had died (HR 1·07, 95% CI 0·86-1·32; p=0·56). We found no evidence of interactions between these randomisations or with transfusion randomisations (p>0·2). By day 180, 489 (24%) children in the multivitamin multimineral supplement group versus 509 (26%) children in the iron and folate group (HR 0·95, 95% CI 0·84-1·07; p=0·40), and 500 (25%) children in the co-trimoxazole group versus 498 (25%) children in the no co-trimoxazole group (1·01, 0·89-1·15; p=0·85) had had one or more serious adverse events. Most serious adverse events were re-admissions, occurring in 692 (17%) children (175 [4%] with at least two re-admissions).
Interpretation: Neither enhanced supplementation with multivitamin multimineral supplement versus iron and folate treatment or co-trimoxazole prophylaxis improved 6-month survival. High rates of hospital re-admission suggest that novel interventions are urgently required for severe anaemia, given the burden it places on overstretched health services in Africa.
Funding: Medical Research Council and Department for International Development.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Sickle cell disease in Africa: an urgent need for longitudinal cohort studies.Lancet Glob Health. 2019 Oct;7(10):e1310-e1311. doi: 10.1016/S2214-109X(19)30364-X. Epub 2019 Aug 23. Lancet Glob Health. 2019. PMID: 31451442 Free PMC article. No abstract available.
-
Seeking interventions to reduce post-discharge mortality among children in sub-Saharan Africa.Lancet Glob Health. 2019 Oct;7(10):e1306-e1307. doi: 10.1016/S2214-109X(19)30373-0. Lancet Glob Health. 2019. PMID: 31537352 No abstract available.
References
-
- Stevens GA, Finucane MM, De-Regil LM. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. - PMC - PubMed
-
- Calis JC, Phiri KS, Faragher EB. Severe anemia in Malawian children. N Engl J Med. 2008;358:888–899. - PubMed
-
- WHO . World Health Organization; Geneva: 2013. Pocket book of hospital care for children: second edition Guidelines for the management of common childhood illnesses. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12023/17/MRC_/Medical Research Council/United Kingdom
- MC_PC_MR/R019258/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12023/26/MRC_/Medical Research Council/United Kingdom
- MR/J012483/1/MRC_/Medical Research Council/United Kingdom
- MC_U122886353/MRC_/Medical Research Council/United Kingdom

