Medroxyprogesterone acetate alters the vaginal microbiota and microenvironment in women and increases susceptibility to HIV-1 in humanized mice
- PMID: 31537512
- PMCID: PMC6826019
- DOI: 10.1242/dmm.039669
Medroxyprogesterone acetate alters the vaginal microbiota and microenvironment in women and increases susceptibility to HIV-1 in humanized mice
Abstract
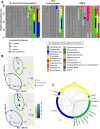
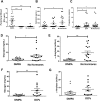
The hormonal contraceptive medroxyprogesterone acetate (MPA) is associated with increased risk of human immunodeficiency virus (HIV), via incompletely understood mechanisms. Increased diversity in the vaginal microbiota modulates genital inflammation and is associated with increased HIV-1 acquisition. However, the effect of MPA on diversity of the vaginal microbiota is relatively unknown. In a cohort of female Kenyan sex workers, negative for sexually transmitted infections (STIs), with Nugent scores <7 (N=58 of 370 screened), MPA correlated with significantly increased diversity of the vaginal microbiota as assessed by 16S rRNA gene sequencing. MPA was also significantly associated with decreased levels of estrogen in the plasma, and low vaginal glycogen and α-amylase, factors implicated in vaginal colonization by lactobacilli, bacteria that are believed to protect against STIs. In a humanized mouse model, MPA treatment was associated with low serum estrogen, low glycogen and enhanced HIV-1 susceptibility. The mechanism by which the MPA-mediated changes in the vaginal microbiota may contribute to HIV-1 susceptibility in humans appears to be independent of inflammatory cytokines and/or activated T cells. Altogether, these results suggest MPA-induced hypo-estrogenism may alter key metabolic components that are necessary for vaginal colonization by certain bacterial species including lactobacilli, and allow for greater bacterial diversity in the vaginal microbiota.This article has an associated First Person interview with the first author of the paper.
Keywords: Amylase; DMPA; Glycogen; Humanized mouse; Polymicrobial vaginal microbiota.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Anahtar M. N., Byrne E. H., Doherty K. E., Bowman B. A., Yamamoto H. S., Soumillon M., Padavattan N., Ismail N., Moodley A., Sabatini M. E. et al. (2015). Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42, 965-976. 10.1016/j.immuni.2015.04.019 - DOI - PMC - PubMed
-
- Anipindi V. C., Bagri P., Roth K., Dizzell S. E., Nguyen P. V., Shaler C. R., Chu D. K., Jiménez-Saiz R., Liang H., Swift S. et al. (2016). Estradiol enhances CD4+ T-cell anti-viral immunity by priming vaginal DCs to induce Th17 responses via an IL-1-dependent pathway. PLoS Pathog. 12, e1005589-e1005589 10.1371/journal.ppat.1005589 - DOI - PMC - PubMed
-
- Bahamondes M. V., Castro S., Marchi N. M., Marcovici M., Andrade L. A. L. A., Fernandes A. and Bahamondes L. (2014). Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception 90, 117-122. 10.1016/j.contraception.2014.01.024 - DOI - PubMed
-
- Bartram A. K., Lynch M. D. J., Stearns J. C., Moreno-Hagelsieb G. and Neufeld J. D. (2011). Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end illumina reads. Appl. Environ. Microbiol. 77, 3846-3852. 10.1128/AEM.02772-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

