Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial
- PMID: 31537547
- PMCID: PMC6937406
- DOI: 10.1136/annrheumdis-2019-215396
Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: a randomised, double-blind, placebo-controlled trial
Abstract
Objectives: Open-labelled clinical trials suggested that low-dose IL-2 might be effective in treatment of systemic lupus erythematosus (SLE). A double-blind and placebo-controlled trial is required to formally evaluate the safety and efficacy of low-dose IL-2 therapy.
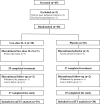
Methods: A randomised, double-blind and placebo-controlled clinical trial was designed to treat 60 patients with active SLE. These patients received either IL-2 (n=30) or placebo (n=30) with standard treatment for 12 weeks, and were followed up for additional 12 weeks. IL-2 at a dose of 1 million IU or placebo was administered subcutaneously every other day for 2 weeks and followed by a 2-week break as one treatment cycle. The primary endpoint was the SLE Responder Index-4 (SRI-4) at week 12. The secondary endpoints were other clinical responses, safety and dynamics of immune cell subsets.
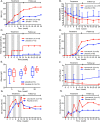
Results: At week 12, the SRI-4 response rates were 55.17% and 30.00% for IL-2 and placebo, respectively (p=0.052). At week 24, the SRI-4 response rate of IL-2 group was 65.52%, compared with 36.67% of the placebo group (p=0.027). The primary endpoint was not met at week 12. Low-dose IL-2 treatment resulted in 53.85% (7/13) complete remission in patients with lupus nephritis, compared with 16.67% (2/12) in the placebo group (p=0.036). No serious infection was observed in the IL-2 group, but two in placebo group. Besides expansion of regulatory T cells, low-dose IL-2 may also sustain cellular immunity with enhanced natural killer cells.
Conclusions: Low-dose IL-2 might be effective and tolerated in treatment of SLE.
Trial registration number: ClinicalTrials.gov Registries (NCT02465580 and NCT02932137).
Keywords: Autoimmune diseases; cytokines; systemic lupus erythematosus; t cells; treatment.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

