A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation
- PMID: 31537642
- PMCID: PMC6888976
- DOI: 10.1084/jem.20182111
A CCL1/CCR8-dependent feed-forward mechanism drives ILC2 functions in type 2-mediated inflammation
Abstract
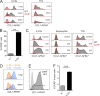
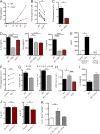
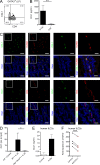
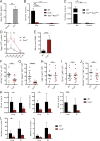
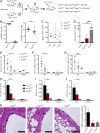
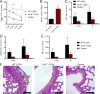
Group 2 innate lymphoid cells (ILC2s) possess indispensable roles during type 2-mediated inflammatory diseases. Although their physiological and detrimental immune functions seem to depend on the anatomical compartment they reside, their tissue tropism and the molecular and immunological processes regulating the self-renewal of the local pool of ILC2s in the context of inflammation or infection are incompletely understood. Here, we analyzed the role of the CC-chemokine receptor CCR8 for the biological functions of ILC2s. In vitro and in vivo experiments indicated that CCR8 is in comparison to the related molecule CCR4 less important for migration of these cells. However, we found that activated mouse and human ILC2s produce the CCR8 ligand CCL1 and are a major source of CCL1 in vivo. CCL1 signaling to ILC2s regulates their proliferation and supports their capacity to protect against helminthic infections. In summary, we identify a novel chemokine receptor-dependent mechanism by which ILC2s are regulated during type 2 responses.
© 2019 Knipfer et al.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

