Structure-based inhibitors halt prion-like seeding by Alzheimer's disease-and tauopathy-derived brain tissue samples
- PMID: 31537646
- PMCID: PMC6827308
- DOI: 10.1074/jbc.RA119.009688
Structure-based inhibitors halt prion-like seeding by Alzheimer's disease-and tauopathy-derived brain tissue samples
Abstract
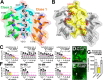
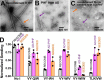
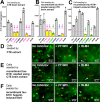
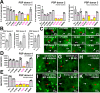
In Alzheimer's disease (AD) and tauopathies, tau aggregation accompanies progressive neurodegeneration. Aggregated tau appears to spread between adjacent neurons and adjacent brain regions by prion-like seeding. Hence, inhibitors of this seeding offer a possible route to managing tauopathies. Here, we report the 1.0 Å resolution micro-electron diffraction structure of an aggregation-prone segment of tau with the sequence SVQIVY, present in the cores of patient-derived fibrils from AD and tauopathies. This structure illuminates how distinct interfaces of the parent segment, containing the sequence VQIVYK, foster the formation of distinct structures. Peptide-based fibril-capping inhibitors designed to target the two VQIVYK interfaces blocked proteopathic seeding by patient-derived fibrils. These VQIVYK inhibitors add to a panel of tau-capping inhibitors that targets specific polymorphs of recombinant and patient-derived tau fibrils. Inhibition of seeding initiated by brain tissue extracts differed among donors with different tauopathies, suggesting that particular fibril polymorphs of tau are associated with certain tauopathies. Donors with progressive supranuclear palsy exhibited more variation in inhibitor sensitivity, suggesting that fibrils from these donors were more polymorphic and potentially vary within individual donor brains. Our results suggest that a subset of inhibitors from our panel could be specific for particular disease-associated polymorphs, whereas inhibitors that blocked seeding by extracts from all of the tauopathies tested could be used to broadly inhibit seeding by multiple disease-specific tau polymorphs. Moreover, we show that tau-capping inhibitors can be transiently expressed in HEK293 tau biosensor cells, indicating that nucleic acid-based vectors can be used for inhibitor delivery.
Keywords: amyloid; crystal structure; fibril; inhibitor; neurodegeneration; prion; protein aggregation; protein structure; seeding; structural biology; tau protein; tauopathy; zipper interface.
© 2019 Seidler et al.
Conflict of interest statement
D. S. E. is SAB chair and equity holder of ADRx, Inc
Figures



References
-
- Goedert M., Wischik C. M., Crowther R. A., Walker J. E., and Klug A. (1988) Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl. Acad. Sci. U.S.A. 85, 4051–4055 10.1073/pnas.85.11.4051 - DOI - PMC - PubMed
-
- Mudher A., Colin M., Dujardin S., Medina M., Dewachter I., Alavi Naini S. M., Mandelkow E.-M., Mandelkow E., Buée L., Goedert M., and Brion J.-P. (2017) What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol. Commun. 5, 99–99 10.1186/s40478-017-0488-7 - DOI - PMC - PubMed
-
- Clavaguera F., Akatsu H., Fraser G., Crowther R. A., Frank S., Hench J., Probst A., Winkler D. T., Reichwald J., Staufenbiel M., Ghetti B., Goedert M., and Tolnay M. (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. U.S.A. 110, 9535–9540 10.1073/pnas.1301175110 - DOI - PMC - PubMed
-
- Sanders D. W., Kaufman S. K., DeVos S. L., Sharma A. M., Mirbaha H., Li A., Barker S. J., Foley A. C., Thorpe J. R., Serpell L. C., Miller T. M., Grinberg L. T., Seeley W. W., and Diamond M. I. (2014) Distinct Tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 10.1016/j.neuron.2014.04.047 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

