Amyloid-Beta Modulates Low-Threshold Activated Voltage-Gated L-Type Calcium Channels of Arcuate Neuropeptide Y Neurons Leading to Calcium Dysregulation and Hypothalamic Dysfunction
- PMID: 31537707
- PMCID: PMC6820205
- DOI: 10.1523/JNEUROSCI.0617-19.2019
Amyloid-Beta Modulates Low-Threshold Activated Voltage-Gated L-Type Calcium Channels of Arcuate Neuropeptide Y Neurons Leading to Calcium Dysregulation and Hypothalamic Dysfunction
Abstract
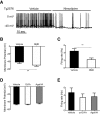
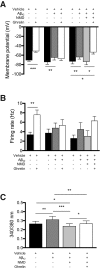
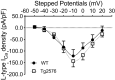
Weight loss is an early manifestation of Alzheimer's disease that can precede the cognitive decline, raising the possibility that amyloid-β (Aβ) disrupts hypothalamic neurons critical for the regulation of body weight. We previously reported that, in young transgenic mice overexpressing mutated amyloid precursor protein (Tg2576), Aβ causes dysfunction in neuropeptide Y (NPY)-expressing hypothalamic arcuate neurons before plaque formation. In this study, we examined whether Aβ causes arcuate NPY neuronal dysfunction by disrupting intracellular Ca2+ homeostasis. Here, we found that the L-type Ca2+ channel blocker nimodipine could hyperpolarize the membrane potential, decrease the spontaneous activity, and reduce the intracellular Ca2+ levels in arcuate NPY neurons from Tg2576 brain slices. In these neurons, there was a shift from high to low voltage-threshold activated L-type Ca2+ currents, resulting in increased Ca2+ influx closer to the resting membrane potential, an effect recapitulated by Aβ1-42 and reversed by nimodipine. These low voltage-threshold activated L-type Ca2+ currents were dependent in part on calcium/calmodulin-dependent protein kinase II and IP3 pathways. Furthermore, the effects on intracellular Ca2+ signaling by both a positive (ghrelin) and negative (leptin) modulator were blunted in these neurons. Nimodipine pretreatment restored the response to ghrelin-mediated feeding in young (3-5 months), but not older (10 months), female Tg2576 mice, suggesting that intracellular Ca2+ dysregulation is only reversible early in Aβ pathology. Collectively, these findings provide evidence for a key role for low-threshold activated voltage gated L-type Ca2+ channels in Aβ-mediated neuronal dysfunction and in the regulation of body weight.SIGNIFICANCE STATEMENT Weight loss is one of the earliest manifestations of Alzheimer's disease (AD), but the underlying cellular mechanisms remain unknown. Disruption of intracellular Ca2+ homeostasis by amyloid-β is hypothesized to be critical for the early neuronal dysfunction driving AD pathogenesis. Here, we demonstrate that amyloid-β causes a shift from high to low voltage-threshold activated L-type Ca2+ currents in arcuate neuropeptide Y neurons. This leads to increased Ca2+ influx closer to the resting membrane potential, resulting in intracellular Ca2+ dyshomeostasis and neuronal dysfunction, an effect reversible by the L-type Ca2+ channel blocker nimodipine early in amyloid-β pathology. These findings highlight a novel mechanism of amyloid-β-mediated neuronal dysfunction through L-type Ca2+ channels and the importance of these channels in the regulation of body weight.
Keywords: Alzheimer's disease; electrophysiology; ghrelin; hypothalamus; leptin; neuropeptide Y.
Copyright © 2019 the authors.
Figures





References
-
- Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, et al. (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661. 10.1016/S0896-6273(03)00063-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
