Characterisation and localisation of the endocannabinoid system components in the adult human testis
- PMID: 31537814
- PMCID: PMC6753062
- DOI: 10.1038/s41598-019-49177-y
Characterisation and localisation of the endocannabinoid system components in the adult human testis
Erratum in
-
Author Correction: Characterisation and localisation of the endocannabinoid system components in the adult human testis.Sci Rep. 2020 Jan 22;10(1):1267. doi: 10.1038/s41598-020-58153-w. Sci Rep. 2020. PMID: 31965051 Free PMC article.
Abstract
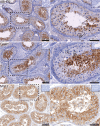
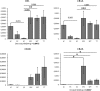
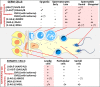
Heavy use of cannabis (marijuana) has been associated with decreased semen quality, which may reflect disruption of the endocannabinoid system (ECS) in the male reproductive tract by exogenous cannabinoids. Components of ECS have been previously described in human spermatozoa and in the rodent testis but there is little information on the ECS expression within the human testis. In this study we characterised the main components of the ECS by immunohistochemistry (IHC) on archived testis tissue samples from 15 patients, and by in silico analysis of existing transcriptome datasets from testicular cell populations. The presence of 2-arachidonoylglycerol (2-AG) in the human testis was confirmed by matrix-assisted laser desorption ionization imaging analysis. Endocannabinoid-synthesising enzymes; diacylglycerol lipase (DAGL) and N-acyl-phosphatidylethanolamine-specific phospholipase D (NAPE-PLD), were detected in germ cells and somatic cells, respectively. The cannabinoid receptors, CNR1 and CNR2 were detected at a low level in post-meiotic germ cells and Leydig- and peritubular cells. Different transcripts encoding distinct receptor isoforms (CB1, CB1A, CB1B and CB2A) were also differentially distributed, mainly in germ cells. The cannabinoid-metabolising enzymes were abundantly present; the α/β-hydrolase domain-containing protein 2 (ABHD2) in all germ cell types, except early spermatocytes, the monoacylglycerol lipase (MGLL) in Sertoli cells, and the fatty acid amide hydrolase (FAAH) in late spermatocytes and post-meiotic germ cells. Our findings are consistent with a direct involvement of the ECS in regulation of human testicular physiology, including spermatogenesis and Leydig cell function. The study provides new evidence supporting observations that recreational cannabis can have possible deleterious effects on human testicular function.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
The endocannabinoid system in human testes.Nat Rev Endocrinol. 2019 Dec;15(12):684-685. doi: 10.1038/s41574-019-0271-x. Nat Rev Endocrinol. 2019. PMID: 31578498 No abstract available.
References
-
- Hembree WC, 3rd, Nahas GG, Zeidenberg P, Huang HF. Changes in human spermatozoa associated with high dose marihuana smoking. Adv. Biosci. 1978;22-23:429–39. - PubMed
-
- Gundersen TD, et al. Association between use of marijuana and male reproductive hormones and semen quality: A study among 1,215 healthy young men. Am. J. Epidemiol. 2015;182:473–481. - PubMed
-
- Hsiao P, Clavijo RI. Adverse effects of Cannabis on male reproduction. Eur Urol. Focus. 2018;4:324–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

