Process development for scale-up production of a therapeutic L-asparaginase by Streptomyces brollosae NEAE-115 from shake flasks to bioreactor
- PMID: 31537817
- PMCID: PMC6753079
- DOI: 10.1038/s41598-019-49709-6
Process development for scale-up production of a therapeutic L-asparaginase by Streptomyces brollosae NEAE-115 from shake flasks to bioreactor
Abstract
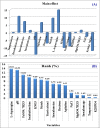
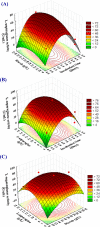
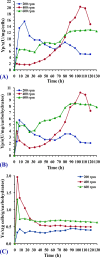
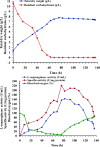
L-asparaginase is a promising enzyme that has a wide range of significant applications including cancer therapy and starchy food industries. The statistical design of Plackett-Burman and face centered central composite design were employed to optimize L-asparaginase production by Streptomyces brollosae NEAE-115. As a result, a medium of the following formula is the optimum for producing L-asparaginase in the culture filtrate of Streptomyces brollosae NEAE-115: Dextrose 2 g, starch 20 g, L-asparagine 10 g, KNO3 1 g, K2HPO4 1 g, MgSO4.7H2O 0.5 g, NaCl 0.1 g, pH 7, fermentation period 7 days, temperature 30 °C, inoculum size 4%, v/v, agitation speed 150 rpm and inoculum age 48 h. The kinetics of cell growth, carbohydrates consumption and L- asparaginase production were studied in 7-L stirred tank bioreactor under different cultivation conditions. A significant increase in both cell growth and carbohydrate consumption was observed as the stirring speed increased from 200 to 600 rpm under uncontrolled pH. The highest L- asparaginase activity of 108.46 U/mL was obtained after 96 h at 400 rpm. On the other hand, the specific enzyme production (Yp/x) under uncontrolled pH reached its maximal value of about 20.3 U/mg cells. Further improvement of enzyme production was attained by controlling pH at 7 using the selected stirring speed of 400 rpm. Enzyme production of 162.11 U/mL obtained from the controlled pH cultures exceeded this value gained from uncontrolled pH (108.46 U/mL) by about 50%.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kumar K, Verma N. The various sources and application of L-asparaginase. Asian J Biochem Pharm Res. 2012;2:197–205.
-
- Duval M, et al. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized european organisation for research and treatment of cancer—children’s leukemia group phase 3 trial. Blood. 2002;99:2734–2739. doi: 10.1182/blood.V99.8.2734. - DOI - PubMed
-
- Savitri AN, Azmi W. Microbial L-asparaginase: A potent antitumour enzyme. Ind J Biotechnol. 2003;2:184–194.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

