Functional organization of motor networks in the lumbosacral spinal cord of non-human primates
- PMID: 31537819
- PMCID: PMC6753145
- DOI: 10.1038/s41598-019-49328-1
Functional organization of motor networks in the lumbosacral spinal cord of non-human primates
Abstract
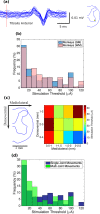
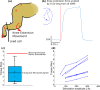
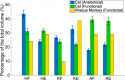
Implantable spinal-cord-neuroprostheses aiming to restore standing and walking after paralysis have been extensively studied in animal models (mainly cats) and have shown promising outcomes. This study aimed to take a critical step along the clinical translation path of these neuroprostheses, and investigated the organization of the neural networks targeted by these implants in a non-human primate. This was accomplished by advancing a microelectrode into various locations of the lumbar enlargement of the spinal cord, targeting the ventral horn of the gray matter. Microstimulation in these locations produced a variety of functional movements in the hindlimb. The resulting functional map of the spinal cord in monkeys was found to have a similar overall organization along the length of the spinal cord to that in cats. This suggests that the human spinal cord may also be organized similarly. The obtained spinal cord maps in monkeys provide important knowledge that will guide the very first testing of these implants in humans.
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

