Expanding the functional group tolerance of cross-coupling in 1,2-dihydro-1,2-azaborines: installation of alkyl, alkenyl, aryl, and heteroaryl substituents while maintaining a B-H bond
- PMID: 31537948
- PMCID: PMC6752210
- DOI: 10.1016/j.tet.2018.12.039
Expanding the functional group tolerance of cross-coupling in 1,2-dihydro-1,2-azaborines: installation of alkyl, alkenyl, aryl, and heteroaryl substituents while maintaining a B-H bond
Abstract
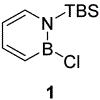
Palladium-catalyzed Negishi cross-coupling of 3-bromo-1-(tert-butyldimethylsilyl)-1,2-dihydro-1,2-azaborine while maintaining the B-H functionality has been demonstrated. 17 examples, including dialkylzinc, alkyl-, alkenyl-, aryl-, as well as nitrogen-, sulfur-, and oxygen-containing heteroaryl-zinc halide reagents have been coupled to generate new C(3) substituted 1,2-azaborines in moderate to excellent yields.
Figures


References
-
-
For an overview of BN/CC isosterism, see:
Morgan MM, Piers WE, Dalton Trans. 45 (2016) 5920.
Belanger-Chabot G, Braunschweig H, Roy DK, Eur. J. Inorg. Chem (2017) 4353.
Giustra ZX, Liu SY, J. Am. Chem. Soc 140 (2018) 1184.
-
-
-
For the initial syntheses of monocyclic 1,2-azaborines, see:
Dewar MJS, Marr PA, J. Am. Chem. Soc 84 (1962), 3782.
White DG, J. Am. Chem. Soc 85 (1963), 3634.
-
-
- Ashe AJ III. Fang, Org. Lett 2 (2000) 2089. - PubMed
- Ashe AJ III., Fang X, Fang X, Kampf JW, Organometallics 20 (2001) 5413.
-
- Pan J, Kampf JW, Ashe AJ III., Org. Lett 9 (2007) 679. - PubMed
-
- Marwitz AJV, Matus MH, Zakharov LN, Dixon DA, Liu S-Y, Angew. Chem. Int. Ed 48 (2009) 973. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
