Scheduled injection of ramosetron for prevention of nausea and vomiting following single-port access total laparoscopic hysterectomy: a prospective randomized study
- PMID: 31538078
- PMCID: PMC6737055
- DOI: 10.5468/ogs.2019.62.5.344
Scheduled injection of ramosetron for prevention of nausea and vomiting following single-port access total laparoscopic hysterectomy: a prospective randomized study
Abstract
Objective: The purpose of this study was to evaluate the effectiveness of scheduled ramosetron injections for controlling postoperative nausea and vomiting (PONV) after single-port access total laparoscopic hysterectomy (SPA-TLH).
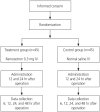
Methods: Ninety patients who underwent SPA-TLH at the Korean National Health Insurance Service Ilsan Hospital between June 2013 and July 2014 were enrolled in this prospective, randomized, double-blinded, placebo-controlled study. The patients were divided into 2 groups as follows: the ramosetron group (0.3 mg intravenously [IV]; n=45) and the placebo group (normal saline IV; n=45). Both groups received their respective injections 12 and 24 hours post surgery. The incidence and severity of PONV (numerical rating scale, 0-10), and the use of rescue antiemetics post surgery were evaluated.
Results: Demographic and perioperative statistically significant differences were not observed between the 2 groups. The incidence of PONV in the ramosetron and placebo groups was 46.7% and 51.1%, respectively (P=0.51). We found significant differences in the severity of PONV between the 24- to 48-hour postoperative periods in both groups (ramosetron group, P=0.04 and placebo group, P=0.03). The use of rescue antiemetics was significantly lower in the ramosetron group than in the placebo group (P=0.02).
Conclusion: After general anesthesia, scheduled injections of ramosetron 12 and 24 hours after SPA-TLH reduced the severity of PONV and the use of rescue antiemetics. Administration of ramosetron can be considered not only immediately after SPA-TLH but also during the first 24-hour recovery period.
Trial registration: ClinicalTrials.gov Identifier: NCT02011659.
Keywords: Hysterectomy; Laparoscopy; Postoperative nausea and vomiting; Ramosetron.
Conflict of interest statement
Conflict of interest: No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89:652–658. - PubMed
-
- Gan TJ, Meyer T, Apfel CC, Chung F, Davis PJ, Eubanks S, et al. Consensus guidelines for managing postoperative nausea and vomiting. Anesth Analg. 2003;97:62–71. - PubMed
-
- Rowbotham D. Recognising risk factors. Nurs Times. 1995;91:44–46. - PubMed
-
- Stadler M, Bardiau F, Seidel L, Albert A, Boogaerts JG. Difference in risk factors for postoperative nausea and vomiting. Anesthesiology. 2003;98:46–52. - PubMed
-
- Jacobson TZ, Davis CJ. Safe laparoscopy: is it possible? Curr Opin Obstet Gynecol. 2004;16:283–288. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical