Improving the Gastrointestinal Tolerability of Fumaric Acid Esters: Early Findings on Gastrointestinal Events with Diroximel Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis from the Phase 3, Open-Label EVOLVE-MS-1 Study
- PMID: 31538304
- PMCID: PMC6822793
- DOI: 10.1007/s12325-019-01085-3
Improving the Gastrointestinal Tolerability of Fumaric Acid Esters: Early Findings on Gastrointestinal Events with Diroximel Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis from the Phase 3, Open-Label EVOLVE-MS-1 Study
Abstract
Introduction: Diroximel fumarate (DRF) is a novel oral fumarate in development for patients with relapsing forms of multiple sclerosis (MS). Clinical findings from the DRF development program suggest that rates of gastrointestinal (GI) treatment-emergent adverse events (TEAEs) and discontinuation due to GI TEAEs are low, based on clinical and real-world observations of other fumaric acid esters, including dimethyl fumarate (DMF). The incidence of GI TEAEs varies from 40 to 88% in clinical and real-world studies of DMF. The objective of this study is to present GI tolerability findings from the EVOLVE-MS-1 study and present biologic hypotheses for the improved GI properties of DRF.
Methods: GI TEAEs and treatment discontinuation because of GI TEAEs were assessed in DRF-treated patients with relapsing-remitting MS who were participating in the ongoing, 96-week, open-label, phase 3 EVOLVE-MS-1 study.
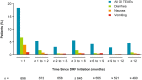
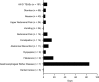
Results: As of March 30, 2018, a total of 696 patients were enrolled in EVOLVE-MS-1. GI TEAEs were reported in 30.9% (215/696) of patients; the vast majority (96%; 207/215) experienced events that were mild or moderate in severity. When GI AEs did occur, they occurred early in treatment, resolved (88.8%; 191/215), and were of short duration [median 7.5 (range 1-87) days] in most patients. GI TEAEs led to < 1% of patients discontinuing treatment.
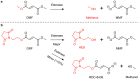
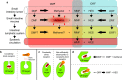
Conclusions: We suggest that the distinct chemical structure of DRF contributes to the observed low rates of GI TEAEs and GI-associated treatment discontinuations, possibly due to a combination of several factors. We hypothesize that these factors may include less reactivity with off-target proteins and/or lower production of a methanol leaving group that may contribute to GI irritation. A direct comparison of GI tolerability with DRF versus DMF is being evaluated in the EVOLVE-MS-2 study.
Trial registration: ClinicalTrials.gov number NCT02634307.
Funding: Alkermes Inc. (Waltham, MA, USA) and Biogen (Cambridge, MA, USA).
Keywords: Dimethyl fumarate; Diroximel fumarate; Fumaric acid ester; Gastrointestinal; Multiple sclerosis; Neurology; Relapsing-remitting multiple sclerosis; Tolerability.
Figures
References
-
- Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing-remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: a multicenter retrospective observational study (STRATEGY) Mult Scler Relat Disord. 2018;22:27–34. doi: 10.1016/j.msard.2018.02.028. - DOI - PubMed
-
- Kresa-Reahl K, Repovic P, Robertson D, Okwuokenye M, Meltzer L, Mendoza JP. Effectiveness of delayed-release dimethyl fumarate on clinical and patient-reported outcomes in patients with relapsing multiple sclerosis switching from glatiramer acetate: RESPOND, a prospective observational study. Clin Ther. 2018;40(12):2077–2087. doi: 10.1016/j.clinthera.2018.10.011. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

