Control and Elimination of Extensively Drug-Resistant Acinetobacter baumanii in an Intensive Care Unit
- PMID: 31538925
- PMCID: PMC6759246
- DOI: 10.3201/eid2510.181626
Control and Elimination of Extensively Drug-Resistant Acinetobacter baumanii in an Intensive Care Unit
Abstract
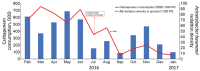
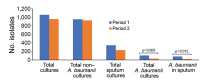
We decreased antimicrobial drug consumption in an intensive care unit in Lebanon by changing to colistin monotherapy for extensively drug-resistant Acinetobacter baumanii infections. We saw a 78% decrease of A. baumanii in sputum and near-elimination of blaoxa-23-carrying sequence type 2 clone over the 1-year study. Non-A. baumanii multidrug-resistant infections remained stable.
Keywords: Acinetobacter baumanii; France; Lebanon; MDR; XDR; antimicrobial resistance; antimicrobial stewardship; bacteria; carbapenem.
Figures
References
-
- Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–9. 10.1016/j.ijantimicag.2009.10.005 - DOI - PubMed
-
- Batirel A, Balkan II, Karabay O, Agalar C, Akalin S, Alici O, et al. Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33:1311–22. 10.1007/s10096-014-2070-6 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

