Negative Hyperselection of Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer Who Received Panitumumab-Based Maintenance Therapy
- PMID: 31539295
- PMCID: PMC6864846
- DOI: 10.1200/JCO.19.01254
Negative Hyperselection of Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer Who Received Panitumumab-Based Maintenance Therapy
Abstract
Purpose: We assessed the prognostic/predictive role of primary tumor sidedness and uncommon alterations of anti-epidermal growth factor receptor (EGFR) primary resistance (primary resistance in RAS and BRAF wild-type metastatic colorectal cancer patients treated with anti-EGFR monoclonal antibodies [PRESSING] panel) in patients with RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC) who were randomly assigned to panitumumab plus fluorouracil, leucovorin, and oxaliplatin (FOLFOX-4) induction followed by maintenance with panitumumab with or without fluorouracil (FU) plus leucovorin (LV); Valentino trial (ClinicalTrials.gov identifier: NCT02476045).
Patients and methods: This prespecified retrospective analysis included 199 evaluable patients with RAS/BRAF wt. The PRESSING panel included the following: immunohistochemistry (IHC) and in situ hybridization for HER2/MET amplification, IHC with or without RNA sequencing for ALK/ROS1/NTRKs/RET fusions, next-generation sequencing for HER2/PIK3CAex.20/PTEN/AKT1 and RAS mutations with low mutant allele fraction, and multiplex polymerase chain reaction for microsatellite instability. PRESSING status (any positive biomarker v all negative) and sidedness were correlated with overall response rate (ORR), progression-free survival (PFS), and overall survival (OS) in the study population and by treatment arm.
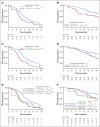
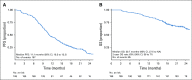
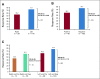
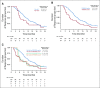
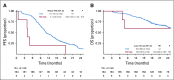
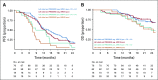
Results: Overall, left- and right-sided tumors were 85.4% and 14.6%, respectively, and PRESSING-negative and -positive tumors were 75.4% and 24.6%, respectively. At a median follow-up of 26 months, inferior outcomes were consistently observed in right- versus left-sided tumors for ORR (55.2% v 74.1%; P = .037), PFS (8.4 v 11.5 months; P = .026), and OS (2-year rate: 50.2% v 65.1%; P = .062). Similar results were observed in the PRESSING-positive versus PRESSING-negative subgroup for ORR (59.2% v 75.3%; P = .030), PFS (7.7 v 12.1 months; P < .001), and OS (2-year rate: 48.1% v 68.1%; P = .021). The PFS benefit of FU plus LV added to panitumumab maintenance, reported in the study, was independent from sidedness and PRESSING status (interaction for PFS P = .293 and .127, respectively). However, outcomes were extremely poor in patients who received single-agent panitumumab and had right-sided tumors (median PFS, 7.7 months; 2-year OS, 38.5%) or PRESSING-positive tumors (median PFS, 7.4 months; 2-year OS, 47.0%).
Conclusion: The combined assessment of sidedness and molecular alterations of anti-EGFR primary resistance identified a consistent proportion of patients with RAS/BRAF-wt mCRC who had inferior benefit from initial anti-EGFR-based regimens, particularly after maintenance with single-agent anti-EGFRs.
Figures


Comment in
-
Negative Predictive Biomarkers in Colorectal Cancer: PRESSING Ahead.J Clin Oncol. 2019 Nov 20;37(33):3066-3068. doi: 10.1200/JCO.19.01977. Epub 2019 Sep 24. J Clin Oncol. 2019. PMID: 31550189 No abstract available.
References
-
- Van Cutsem E, Lenz HJ, Köhne CH, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692–700. - PubMed
-
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. - PubMed
-
- National Comprehensive Cancer Network https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf Colon cancer (version 1.2019).
-
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

