Morphological, microbiological and ultrastructural aspects of sepsis by Aeromonas hydrophila in Piaractus mesopotamicus
- PMID: 31539396
- PMCID: PMC6754153
- DOI: 10.1371/journal.pone.0222626
Morphological, microbiological and ultrastructural aspects of sepsis by Aeromonas hydrophila in Piaractus mesopotamicus
Abstract
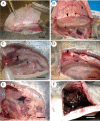
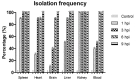
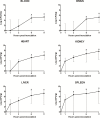
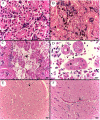
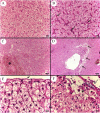
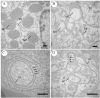
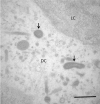
Aeromonas bacteria can cause an infection characterized by septicemia and is one of the most common pathogens in tropical fish. This disease is responsible for high morbidity and mortality rates, causing considerable losses in aquaculture. Thus, the understanding of its pathophysiology is crucial to develop control strategies of this bacterial infection in farmed fish. This study aimed to characterize early pathological aspects of acute sepsis in pacu (Piaractus mesopotamicus) experimentally infected with Aeromonas hydrophila. A total of 160 juvenile pacus were inoculated intraperitoneally with A. hydrophila (1.78 x 109 CFU/mL) and at 0 (control), 1, 3, 6, and 9 hours post-inoculation (hpi), animals were anesthetized and samples were collected for microbiological, light microscopy and transmission electron microscopy (TEM) analyzes. The results showed the occurrence of hemodynamic alterations, such as hemorrhage and congestion, which were observed mainly after 6 and 9 hpi. It was possible to re-isolate Aeromonas at all sampling times except in control group. However, just after 9 hpi it was possible to find the bacteria in all fish and tissues. Light microscopy analyses revealed a degenerative process, necrosis and vascular damage mainly at 6 and 9 hpi. According to the ultrastructural examination, areas of cellular death were identified in all examined tissues, especially at 6 and 9 hpi. However, the most severe, related to necrosis, were observed after 6 and 9 hpi. The findings suggested that this bacterium spreads in the first hpi through the fish organs, mainly affecting spleen, liver and kidney, causing irreversible lesions at the molecular level.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2016. Contributing to food security and nutrition for all. Rome. 2016;200p.
-
- IBGE (Instituto Brasileiro de Geografia e Estatística). Produção Pecuária Municipal. 2015;43:1–49.
-
- Moraes FR, Martins ML. Condições pré-disponentes e principais enfermidades de teleósteos em piscicultura intensiva In: Cyrino JEP, Urbinati EC, Fracalossi D M, Castangnolli N, editors. Tópicos especiais em piscicultura de água doce tropical intensiva. São Paulo: TecArt; 2004. p. 343–86.
-
- Holliman A. The veterinary approach to trout In: Brown L, editor. Aquaculture for veterinarians: fish husbandry and medicine. Oxford: Pergamon Press; 1993. p. 223–47.
-
- Cyrino JEP, Urbinati EC, Fracalossi DM, Castagnolli N. Tópicos Especiais em Piscicultura de Água Doce Tropical Intensiva. São Paulo: TecArte; 2004. p. 533.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

