Mice deficient in NKLAM have attenuated inflammatory cytokine production in a Sendai virus pneumonia model
- PMID: 31539400
- PMCID: PMC6754162
- DOI: 10.1371/journal.pone.0222802
Mice deficient in NKLAM have attenuated inflammatory cytokine production in a Sendai virus pneumonia model
Abstract
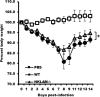
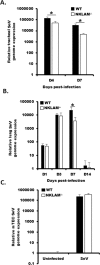
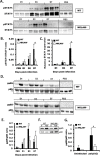
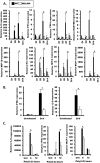
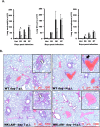
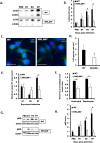
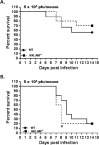
Recent studies have begun to elucidate a role for E3 ubiquitin ligases as important mediators of the innate immune response. Our previous work defined a role for the ubiquitin ligase natural killer lytic-associated molecule (NKLAM/RNF19b) in mouse and human innate immunity. Here, we present novel data describing a role for NKLAM in regulating the immune response to Sendai virus (SeV), a murine model of paramyxoviral pneumonia. NKLAM expression was significantly upregulated by SeV infection. SeV-infected mice that are deficient in NKLAM demonstrated significantly less weight loss than wild type mice. In vivo, Sendai virus replication was attenuated in NKLAM-/- mice. Autophagic flux and the expression of autophagy markers LC3 and p62/SQSTM1 were also less in NKLAM-/- mice. Using flow cytometry, we observed less neutrophils and macrophages in the lungs of NKLAM-/- mice during SeV infection. Additionally, phosphorylation of STAT1 and NFκB p65 was lower in NKLAM-/- than wild type mice. The dysregulated phosphorylation profile of STAT1 and NFκB in NKLAM-/- mice correlated with decreased expression of numerous proinflammatory cytokines that are regulated by STAT1 and/or NFκB. The lack of NKLAM and the resulting attenuated immune response is favorable to NKLAM-/- mice receiving a low dose of SeV; however, at a high dose of virus, NKLAM-/- mice succumbed to the infection faster than wild type mice. In conclusion, our novel results indicate that NKLAM plays a role in regulating the production of pro-inflammatory cytokines during viral infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Reduced inflammation and cytokine production in NKLAM deficient mice during Streptococcus pneumoniae infection.PLoS One. 2018 Mar 8;13(3):e0194202. doi: 10.1371/journal.pone.0194202. eCollection 2018. PLoS One. 2018. PMID: 29518136 Free PMC article.
-
E3 ubiquitin ligase NKLAM positively regulates macrophage inducible nitric oxide synthase expression.Immunobiology. 2015 Jan;220(1):83-92. doi: 10.1016/j.imbio.2014.08.016. Epub 2014 Aug 23. Immunobiology. 2015. PMID: 25182373 Free PMC article.
-
E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity.Cell Signal. 2016 Dec;28(12):1833-1841. doi: 10.1016/j.cellsig.2016.08.014. Epub 2016 Aug 26. Cell Signal. 2016. PMID: 27570112 Free PMC article.
-
Natural Killer Lytic-Associated Molecule (NKLAM): An E3 Ubiquitin Ligase With an Integral Role in Innate Immunity.Front Physiol. 2020 Oct 29;11:573372. doi: 10.3389/fphys.2020.573372. eCollection 2020. Front Physiol. 2020. PMID: 33192571 Free PMC article. Review.
-
[Sendai virus proteins counteracting the host innate immunity].Uirusu. 2004 Dec;54(2):179-88. doi: 10.2222/jsv.54.179. Uirusu. 2004. PMID: 15745155 Review. Japanese.
Cited by
-
Identification of common genetic characteristics of rheumatoid arthritis and major depressive disorder by bioinformatics analysis and machine learning.Front Immunol. 2023 Jun 21;14:1183115. doi: 10.3389/fimmu.2023.1183115. eCollection 2023. Front Immunol. 2023. PMID: 37415981 Free PMC article.
-
Chemoprevention effect of the Mediterranean diet on colorectal cancer: Current studies and future prospects.Front Nutr. 2022 Aug 4;9:924192. doi: 10.3389/fnut.2022.924192. eCollection 2022. Front Nutr. 2022. PMID: 35990343 Free PMC article. Review.
-
Species-Specificity of Transcriptional Regulation and the Response to Lipopolysaccharide in Mammalian Macrophages.Front Cell Dev Biol. 2020 Jul 21;8:661. doi: 10.3389/fcell.2020.00661. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793601 Free PMC article.
-
DIRAS3 enhances RNF19B-mediated RAC1 ubiquitination and degradation in non-small-cell lung cancer cells.iScience. 2023 Jun 16;26(7):107157. doi: 10.1016/j.isci.2023.107157. eCollection 2023 Jul 21. iScience. 2023. PMID: 37485351 Free PMC article.
-
RAB5C, SYNJ1, and RNF19B promote male ankylosing spondylitis by regulating immune cell infiltration.Ann Transl Med. 2021 Jun;9(12):1011. doi: 10.21037/atm-21-2721. Ann Transl Med. 2021. PMID: 34277811 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

