Efficacy of steroid-eluting stents in management of chronic rhinosinusitis after endoscopic sinus surgery: updated meta-analysis
- PMID: 31539461
- PMCID: PMC6901756
- DOI: 10.1002/alr.22443
Efficacy of steroid-eluting stents in management of chronic rhinosinusitis after endoscopic sinus surgery: updated meta-analysis
Abstract
Background: Recently, there has been mounting evidence suggesting the efficacy of steroid-eluting stents (SES) for management of chronic rhinosinusitis after endoscopic sinus surgery (ESS). This meta-analysis serves to evaluate the efficacy of SES in improving postoperative outcomes after ESS.
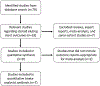
Methods: A systematic literature search was performed of PubMed for articles published between 1985 and 2018. The outcome variables were reported at, on average, 30 days postintervention.
Results: Seven of the 76 published studies, all of which were industry-sponsored, were included for a collective cohort of 444 SES and 444 control sinuses. In patients who received SES vs controls, collective odds ratios (ORs) for postoperative need for intervention, surgery, and oral steroid were 0.45 (95% confidence interval [CI], 0.33-0.62; p < 0.001), 0.30 (95% CI, 0.18-0.52; p < 0.001), and 0.58 (95% CI, 0.40-0.84; p = 0.004), respectively. In addition, collective ORs for frontal sinus ostia (FSO) patency, moderate-to-severe adhesion/scarring, and increase in polyp score were 2.53 (95% CI, 1.61-3.97; p < 0.001), 0.28 (95% CI, 0.13-0.59; p < 0.001), and 0.42 (95% CI, 0.25-0.74; p = 0.002), respectively. Collective mean differences for FSO/ethmoid inflammation and FSO diameter were -10.86 mm (p < 0.001) and +1.34 mm (p < 0.001), respectively.
Conclusion: Aggregate evidence suggests that SES can improve ESS outcomes by reducing rates of postoperative intervention and recurrent polyposis and inflammation, while promoting FSO patency. All included and analyzed studies were industry-sponsored and ruling-out publication bias was not possible. Future independent and nonsponsored studies to further evaluate SES's long-term efficacy are warranted.
Keywords: chronic rhinosinusitis; endoscopic sinus surgery; meta-analysis; steroid-eluting stent.
© 2019 ARS-AAOA, LLC.
Conflict of interest statement
Figures


Similar articles
-
[Studies on efficacy of a bioabsorbable steroid-eluting sinus stent in the frontal sinus opening of chronic rhinosinusitis with nasal polyps].Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021 Aug 7;56(8):824-829. doi: 10.3760/cma.j.cn115330-20200809-00655. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021. PMID: 34521166 Clinical Trial. Chinese.
-
The impact of middle meatal steroid-eluting implants on the postoperative outcomes of chronic rhinosinusitis: A systematic review and meta-analysis.Eur Ann Otorhinolaryngol Head Neck Dis. 2025 Jan;142(1):26-37. doi: 10.1016/j.anorl.2024.02.014. Epub 2024 Mar 22. Eur Ann Otorhinolaryngol Head Neck Dis. 2025. PMID: 38521652
-
Comparison of Bioabsorbable Steroid-Eluting Sinus Stents Versus Nasopore After Endoscopic Sinus Surgery: A Multicenter, Randomized, Controlled, Single-Blinded Clinical Trial.Ear Nose Throat J. 2022 May;101(4):260-267. doi: 10.1177/0145561320947632. Epub 2020 Aug 26. Ear Nose Throat J. 2022. PMID: 32845808 Clinical Trial.
-
Topical therapies for management of chronic rhinosinusitis: steroid implants.Int Forum Allergy Rhinol. 2019 May;9(S1):S22-S26. doi: 10.1002/alr.22344. Int Forum Allergy Rhinol. 2019. PMID: 31087636 Review.
-
Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent.Int Forum Allergy Rhinol. 2011 Jan-Feb;1(1):23-32. doi: 10.1002/alr.20020. Epub 2011 Feb 8. Int Forum Allergy Rhinol. 2011. PMID: 22287304 Clinical Trial.
Cited by
-
Sinonasal Stent Coated with Sustained-Release Varnish of Mometasone Furoate Inhibits Pro-Inflammatory Cytokine Release from Macrophages: An In Vitro Study.Pharmaceutics. 2023 Mar 22;15(3):1015. doi: 10.3390/pharmaceutics15031015. Pharmaceutics. 2023. PMID: 36986875 Free PMC article.
-
The efficacy of steroid-eluting stents on the local inflammation of chronic rhinosinusitis with nasal polyposis after endoscopic sinus surgery: a multicenter prospective longitudinal study.Eur Arch Otorhinolaryngol. 2023 Dec;280(12):5417-5431. doi: 10.1007/s00405-023-08158-8. Epub 2023 Sep 4. Eur Arch Otorhinolaryngol. 2023. PMID: 37665343
-
Safety and Feasibility of Steroid-Eluting Stent as a Bolster in Endoscopic Anterior Skull Base Reconstruction.Ann Otol Rhinol Laryngol. 2024 Jan;133(1):43-49. doi: 10.1177/00034894231181178. Epub 2023 Jun 19. Ann Otol Rhinol Laryngol. 2024. PMID: 37334915 Free PMC article.
-
Editorial Commentary: Association of Comorbid Asthma and the Efficacy of Bioabsorbable Steroid-eluting Sinus Stents Implanted after Endoscopic Sinus Surgery in Patients with Chronic Rhinosinusitis with Nasal Polyps.Curr Med Sci. 2023 Dec;43(6):1258-1259. doi: 10.1007/s11596-023-2826-2. Curr Med Sci. 2023. PMID: 38153632 No abstract available.
-
Management of pott's puffy tumor: a Delphi-method international clinical consensus statement.Eur Arch Otorhinolaryngol. 2025 Aug 9. doi: 10.1007/s00405-025-09589-1. Online ahead of print. Eur Arch Otorhinolaryngol. 2025. PMID: 40782155
References
-
- Pleis JR, Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2006. Vital Health Stat 10. 2007; 235: 1–153. - PubMed
-
- Halawi AM, Smith SS, Chandra RK. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc. 2013; 34(4): 328–334. - PubMed
-
- Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007; 137(3 Suppl): S1–31. - PubMed
-
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995; 113(1):104–109. - PubMed
-
- Murphy MP, Fishman P, Short SO, Sullivan SD, Yueh B, Weymuller EA. Health care utilization and cost among adults with chronic rhinosinusitis enrolled in a health maintenance organization. Otolaryngol Head Neck Surg. 2002; 127(5): 367–376. - PubMed

