Perfused murine heart optical transmission spectroscopy using optical catheter and integrating sphere: Effects of ischemia/reperfusion
- PMID: 31539522
- PMCID: PMC6915838
- DOI: 10.1016/j.ab.2019.113443
Perfused murine heart optical transmission spectroscopy using optical catheter and integrating sphere: Effects of ischemia/reperfusion
Abstract
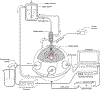
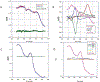
Tissue transmission optical absorption spectroscopy provides dynamic information on metabolism and function. Murine genetic malleability makes it a major model for heart research. The diminutive size of the mouse heart makes optical transmission studies challenging. Using a perfused murine heart center mounted in an integrating sphere for light collection with a ventricular cavity optical catheter as an internal light source provided an effective method of optical data collection in this model. This approach provided high signal to noise optical spectra which when fit with model spectra provided information on tissue oxygenation and redox state. This technique was applied to the study of cardiac ischemia and ischemia reperfusion which generates extreme heart motion, especially during the ischemic contracture. The integrating sphere reduced motion artifacts associated with a fixed optical pickup and methods were developed to compensate for changes in tissue thickness. During ischemia, rapid decreases in myoglobin oxygenation occurred along with increases in cytochrome reduction levels. Surprisingly, when ischemic contracture occurred, myoglobin remained fully deoxygenated, while the cytochromes became more reduced consistent with a further, and critical, reduction of mitochondrial oxygen tension during ischemic contraction. This optical arrangement is an effective method of monitoring murine heart metabolism.
Keywords: Cytochromes; Linear least squares fitting; Mitochondria membrane potential; Myoglobin; Optical pathlength; Oxidative phosphorylation; Oxygen.
Copyright © 2019. Published by Elsevier Inc.
Figures


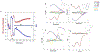
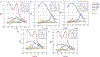
References
-
- Keilin D, On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants, Proc.R.Soc.Lond B Biol.Sci, 98 (1925) 312–339.
-
- Wittenberg BA, Wittenberg JB, Transport of oxygen in musle, Anu.Rev.Physiol, 51 (1989) 857–878. - PubMed
-
- Tamura M, Oshino N, Chance B, Silver IA, Optical measurements of intracellular oxygen concentrations of rat heart in vitro, Arch.Biochem.Biophys, 191 (1978) 18–22. - PubMed
-
- Arai AE, Kasserra CE, Territo PR, Gandjbakhche AH, Balaban RS, Myocardial oxygenation in vivo: optical spectroscopy of cytoplasmic myoglobin and mitochondrial cytochromes, Am.J.Physiol, 277 (1999) H683–H697. - PubMed
-
- Heineman FW, Kupriyanov VV, Marshall R, Fralix TA, Balaban RS, Myocardial oxygenation in the isolated working rabbit heart as a function of work, Am.J.Physiol, 262 (1992) H255–H267. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

