Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress
- PMID: 31539899
- PMCID: PMC6969179
- DOI: 10.1038/s41386-019-0526-1
Accelerated development of cocaine-associated dopamine transients and cocaine use vulnerability following traumatic stress
Abstract
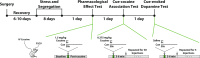
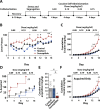
Post-traumatic stress disorder and cocaine use disorder are highly co-morbid psychiatric conditions. The onset of post-traumatic stress disorder generally occurs prior to the development of cocaine use disorder, and thus it appears that the development of post-traumatic stress disorder drives cocaine use vulnerability. We recently characterized a rat model of post-traumatic stress disorder with segregation of rats as susceptible and resilient based on anxiety-like behavior in the elevated plus maze and context avoidance. We paired this model with in vivo fast scan cyclic voltammetry in freely moving rats to test for differences in dopamine signaling in the nucleus accumbens core at baseline, in response to a single dose of cocaine, and in response to cocaine-paired cues. Further, we examined differences in the acquisition of cocaine self-administration across groups. Results indicate that susceptibility to traumatic stress is associated with alterations in phasic dopamine signaling architecture that increase the rate at which dopamine signals entrain to cocaine-associated cues and increase the magnitude of persistent cue-evoked dopamine signals following training. These changes in phasic dopamine signaling correspond with increases in the rate at which susceptible rats develop excessive cocaine-taking behavior. Together, our studies demonstrate that susceptibility to traumatic stress is associated with a cocaine use-vulnerable phenotype and suggests that differences in phasic dopamine signaling architecture may contribute to the process by which this vulnerability occurs.
Figures



References
-
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. - PubMed
-
- Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without post-traumatic stress disorder: a comparison of substance use, trauma history and psychiatric comorbidity. Am J Addict. 2000;9:51–62. - PubMed
-
- Chilcoat HD, Breslau N. Posttraumatic stress disorder and drug disorders: testing causal pathways. Arch Gen Psychiatry. 1998;55:913–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

