The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis
- PMID: 31539914
- PMCID: PMC6698662
- DOI: 10.1093/eurheartj/ehz303
The novel coronary artery disease risk gene JCAD/KIAA1462 promotes endothelial dysfunction and atherosclerosis
Abstract
Aims: Recent genome-wide association studies (GWAS) have identified that the JCAD locus is associated with risk of coronary artery disease (CAD) and myocardial infarction (MI). However, the mechanisms whereby candidate gene JCAD confers disease risk remain unclear. We addressed whether and how JCAD affects the development of atherosclerosis, the common cause of CAD.
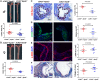
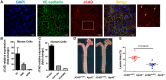
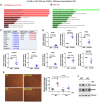
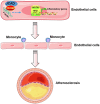
Methods and results: By mining data in the Genotype-Tissue Expression (GTEx) database, we found that CAD-associated risk variants at the JCAD locus are linked to increased JCAD gene expression in human arteries, implicating JCAD as a candidate causal CAD gene. We therefore generated global and endothelial cell (EC) specific-JCAD knockout mice, and observed that JCAD deficiency attenuated high fat diet-induced atherosclerosis in ApoE-deficient mice. JCAD-deficiency in mice also improved endothelium-dependent relaxation. Genome-wide transcriptional profiling of JCAD-depleted human coronary artery ECs showed that JCAD depletion inhibited the activation of YAP/TAZ pathway, and the expression of downstream pro-atherogenic genes, including CTGF and Cyr61. As a result, JCAD-deficient ECs attracted fewer monocytes in response to lipopolysaccharide (LPS) stimulation. Moreover, JCAD expression in ECs was decreased under unidirectional laminar flow in vitro and in vivo. Proteomics studies suggest that JCAD regulates YAP/TAZ activation by interacting with actin-binding protein TRIOBP, thereby stabilizing stress fiber formation. Finally, we observed that endothelial JCAD expression was increased in mouse and human atherosclerotic plaques.
Conclusion: The present study demonstrates that the GWAS-identified CAD risk gene JCAD promotes endothelial dysfunction and atherosclerosis, thus highlighting the possibility of new therapeutic strategies for CAD by targeting JCAD.
Keywords: Atherosclerosis; Coronary artery disease; Endothelial function; GWAS; JCAD/KIAA1462; Therapeutic target.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures






Comment in
-
JCAD: from systems genetics identification to the experimental validation of a coronary artery disease risk locus.Eur Heart J. 2019 Aug 1;40(29):2409-2412. doi: 10.1093/eurheartj/ehz370. Eur Heart J. 2019. PMID: 31539913 No abstract available.
References
-
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–e603. - PMC - PubMed
-
- Atlas Writing G, Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P.. European Society of Cardiology: cardiovascular Disease Statistics 2017. Eur Heart J 2018;39:508–579. - PubMed
-
- Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwoger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ.. ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873–2926. - PubMed
-
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. - PMC - PubMed
-
- Kwak BR, Back M, Bochaton-Piallat ML, Caligiuri G, Daemen MJ, Davies PF, Hoefer IE, Holvoet P, Jo H, Krams R, Lehoux S, Monaco C, Steffens S, Virmani R, Weber C, Wentzel JJ, Evans PC.. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur Heart J 2014;35:3013–20, 3020a-3020d. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

