Hematological Malignancies and HBV Reactivation Risk: Suggestions for Clinical Management
- PMID: 31540124
- PMCID: PMC6784078
- DOI: 10.3390/v11090858
Hematological Malignancies and HBV Reactivation Risk: Suggestions for Clinical Management
Abstract
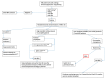
It is well known that hepatitis B virus reactivation (HBVr) can occur among patients undergoing treatment for hematological malignancies (HM). The evaluation of HBVr risk in patients undergoing immunosuppressive treatments is a multidimensional process, which includes conducting an accurate clinical history and physical examination, consideration of the virological categories, of the medication chosen to treat these hematological malignancies and the degree of immunosuppression induced. Once the risk of reactivation has been defined, it is crucial to adopt adequate management strategies (should reactivation occur). The purpose of treatment is to prevent dire clinical consequences of HBVr such as acute/fulminant hepatitis, and liver failure. Treatment will be instituted according to the indications and evidence provided by current international recommendations and to prevent interruption of lifesaving anti-neoplastic treatments. In this paper, we will present the available data regarding the risk of HBVr in this special population of immunosuppressed patients and explore the relevance of effective prevention and management of this potentially life-threatening event. A computerized literature search was performed using appropriate terms to discover relevant articles. Current evidence supports the policy of universal HBV testing of patients scheduled to undergo treatment for hematological malignancies, and clinicians should be aware of the inherent risk of viral reactivation among the different virological categories and classes of immunosuppressive drugs.
Keywords: hematology; hepatitis B virus; immunosuppressive therapy; lymphoma; occult/active/inactive carrier; prophylaxis; reactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

