Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep
- PMID: 31540219
- PMCID: PMC6769582
- DOI: 10.3390/ijms20184530
Immunohistochemical Examination of Trophoblast Syncytialization during Early Placentation in Sheep
Abstract
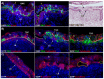
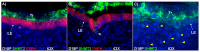
During the peri-implantation period, multinucleated syncytia are formed in the sheep placenta. For over 20 years the scientific consensus has been that during trophoblast syncytialization in sheep, binucleate trophoblast giant cells (BNCs) differentiate from mononuclear trophoblast cells, and individual BNCs fuse with individual luminal epithelial (LE) cells to form trinucleate cells. These trophoblast-LE syncytial plaques then grow through continued BNC migration and fusion. Therefore, LE cells are thought to be incorporated into syncytial plaques. However, these ideas were based on electron microscopy studies, without benefit of molecular markers for BNC and LE cells to support conclusions. The aim of this study was to observe interactions between BNCs and uterine LE cells using immunohistochemical localization for molecular markers for BNCs and uterine LE cells. We performed immunofluorescence staining, laser capture microdissection, and TUNEL staining on the uterine-placental tissues of sheep during early placentation. We observed: (1) syncytial cells containing more than two nuclei within the trophoblast cell layer; (2) depolarized LE cells that express caspase 3 and stain positively for TUNEL; (3) engulfment of caspase 3-positive LE cells by trophoblast giant cells (TGCs) and empty spaces within the LE layer at sites of implantation; (4) rapid enlargement of syncytial plaques; and (5) E-cadherin and TUNEL-positive cells within the uterine stroma underlying degenerating LE was coincident with accumulation of CD45-positive cells at these sites. These data suggest that during early placentation: (1) fusion between trophoblasts is not limited to the formation of BNCs, and the term 'trophoblast giant cell (TGC)' may be appropriate; (2) LE cells undergo apoptosis; (3) apoptotic LE cells are eliminated by TGCs; (4) fusion is not limited to the incorporation of new BNCs but involves the lateral fusion between growing syncytial plaques; and (5) TGCs carry apoptotic LE cells away from the uterine-placental interface for elimination by immune cells within the stroma. These data indicate that uterine LE cells are not incorporated into syncytial plaques, but are engulfed and eliminated, and that early placentation in sheep is more similar to early placentation in humans than is currently understood in that both develop mononucleated cytotrophoblast and multinucleated syncytiotrophoblast layers of entirely placental origin. The elimination of LE cells by sheep TGCs might provide insights into elimination and penetration of LE cells during human embryo implantation.
Keywords: sheep; syncytialization; trophoblast; uterine luminal epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

