A Novel CXCR4 Targeting Protein SDF-1/54 as an HIV-1 Entry Inhibitor
- PMID: 31540474
- PMCID: PMC6783869
- DOI: 10.3390/v11090874
A Novel CXCR4 Targeting Protein SDF-1/54 as an HIV-1 Entry Inhibitor
Abstract
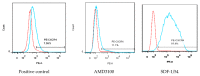
CXC chemokine receptor 4 (CXCR4) is a co-receptor for HIV-1 entry into target cells. Its natural ligand, the chemokine SDF-1, inhibits viral entry mediated by this receptor. However, the broad expression pattern of CXCR4 and its critical roles in various physiological and pathological processes indicate that the direct application of SDF-1 as an entry inhibitor might have severe consequences. Previously, we constructed an effective SDF-1 mutant, SDF-1/54, by deleting the α-helix of the C-terminal functional region of SDF-1. Of note, SDF-1/54 shows remarkable decreased chemotoxic ability, but maintains a similar binding affinity to CXCR4, suggesting SDF-1/54 might better serve as a CXCR4 inhibitor. Here, we found that SDF-1/54 exhibited potent antiviral activity against various X4 HIV-1 strains, including the infectious clone HIV-1 NL4-3, laboratory-adapted strain HIV-1 IIIB, clinical isolates and even drug-resistant strains. By using time-of-addition assay, non-infectious and infectious cell-cell fusion assay and CXCR4 internalization assay, we demonstrated SDF-1/54 is an HIV-1 entry inhibitor. A combination of SDF-1/54 with several antiretroviral drugs exhibited potent synergistic anti-HIV-1 activity. Moreover, SDF-1/54 was stable and its anti-HIV-1 activity was not significantly affected by the presence of seminal fluid, vaginal fluid simulant and human serum albumin. SDF-1/54 showed limited in vitro cytotoxicity to lymphocytes and vaginal epithelial cells. Based on these findings, SDF-1/54 could have a therapeutic potential as an HIV-1 entry inhibitor.
Keywords: CXCR4; HIV-1; SDF-1/54; entry inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Unaids Data 2018. UNAIDS; Geneva, Switzerland: 2018. [(accessed on 16 September 2019)]. Available online: http://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_e....
-
- Cervia J.S., Smith M.A. Enfuvirtide (T-20): a novel human immunodeficiency virus type 1 fusion inhibitor. Clin. Infect. Dis. 2003;37:1102–1106. - PubMed
-
- Schuitemaker H., Koot M., Kootstra N.A., Dercksen M.W., de Goede R.E., van Steenwijk R.P., Lange J.M., Schattenkerk J.K., Miedema F., Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T–cell-tropic virus population. J. Virol. 1992;66:1354–1360. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

