Cellular Stress Responses in Radiotherapy
- PMID: 31540530
- PMCID: PMC6769573
- DOI: 10.3390/cells8091105
Cellular Stress Responses in Radiotherapy
Abstract
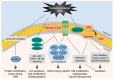
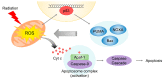
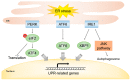
Radiotherapy is one of the major cancer treatment strategies. Exposure to penetrating radiation causes cellular stress, directly or indirectly, due to the generation of reactive oxygen species, DNA damage, and subcellular organelle damage and autophagy. These radiation-induced damage responses cooperatively contribute to cancer cell death, but paradoxically, radiotherapy also causes the activation of damage-repair and survival signaling to alleviate radiation-induced cytotoxic effects in a small percentage of cancer cells, and these activations are responsible for tumor radio-resistance. The present study describes the molecular mechanisms responsible for radiation-induced cellular stress response and radioresistance, and the therapeutic approaches used to overcome radioresistance.
Keywords: DNA damage response; ER stress; Radiation response; autophagy; lipid peroxidation; mitochondrial damage; radioresistance; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

