FGF23-Mediated Activation of Local RAAS Promotes Cardiac Hypertrophy and Fibrosis
- PMID: 31540546
- PMCID: PMC6770314
- DOI: 10.3390/ijms20184634
FGF23-Mediated Activation of Local RAAS Promotes Cardiac Hypertrophy and Fibrosis
Abstract
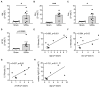
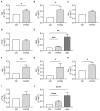
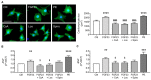
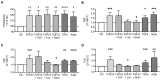
Patients with chronic kidney disease (CKD) are prone to developing cardiac hypertrophy and fibrosis, which is associated with increased fibroblast growth factor 23 (FGF23) serum levels. Elevated circulating FGF23 was shown to induce left ventricular hypertrophy (LVH) via the calcineurin/NFAT pathway and contributed to cardiac fibrosis by stimulation of profibrotic factors. We hypothesized that FGF23 may also stimulate the local renin-angiotensin-aldosterone system (RAAS) in the heart, thereby further promoting the progression of FGF23-mediated cardiac pathologies. We evaluated LVH and fibrosis in association with cardiac FGF23 and activation of RAAS in heart tissue of 5/6 nephrectomized (5/6Nx) rats compared to sham-operated animals followed by in vitro studies with isolated neonatal rat ventricular myocytes and fibroblast (NRVM, NRCF), respectively. Uremic rats showed enhanced cardiomyocyte size and cardiac fibrosis compared with sham. The cardiac expression of Fgf23 and RAAS genes were increased in 5/6Nx rats and correlated with the degree of cardiac fibrosis. In NRVM and NRCF, FGF23 stimulated the expression of RAAS genes and induced Ngal indicating mineralocorticoid receptor activation. The FGF23-mediated hypertrophic growth of NRVM and induction of NFAT target genes were attenuated by cyclosporine A, losartan and spironolactone. In NRCF, FGF23 induced Tgfb and Ctgf, which were suppressed by losartan and spironolactone, only. Our data suggest that FGF23-mediated activation of local RAAS in the heart promotes cardiac hypertrophy and fibrosis.
Keywords: cardiac fibrosis; chronic kidney disease; fibroblast growth factor 23; left ventricular hypertrophy; renin-angiotensin-aldosterone system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Isakova T., Wahl P., Vargas G.S., Gutiérrez O.M., Scialla J., Xie H., Appleby D., Nessel L., Bellovich K., Chen J., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. doi: 10.1038/ki.2011.47. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

