Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial
- PMID: 31540791
- PMCID: PMC6838670
- DOI: 10.1016/S1470-2045(19)30569-8
Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial
Abstract
Background: Localised prostate cancer is commonly treated with external-beam radiotherapy. Moderate hypofractionation has been shown to be non-inferior to conventional fractionation. Ultra-hypofractionated stereotactic body radiotherapy would allow shorter treatment courses but could increase acute toxicity compared with conventionally fractionated or moderately hypofractionated radiotherapy. We report the acute toxicity findings from a randomised trial of standard-of-care conventionally fractionated or moderately hypofractionated radiotherapy versus five-fraction stereotactic body radiotherapy for low-risk to intermediate-risk localised prostate cancer.
Methods: PACE is an international, phase 3, open-label, randomised, non-inferiority trial. In PACE-B, eligible men aged 18 years and older, with WHO performance status 0-2, low-risk or intermediate-risk prostate adenocarcinoma (Gleason 4 + 3 excluded), and scheduled to receive radiotherapy were recruited from 37 centres in three countries (UK, Ireland, and Canada). Participants were randomly allocated (1:1) by computerised central randomisation with permuted blocks (size four and six), stratified by centre and risk group, to conventionally fractionated or moderately hypofractionated radiotherapy (78 Gy in 39 fractions over 7·8 weeks or 62 Gy in 20 fractions over 4 weeks, respectively) or stereotactic body radiotherapy (36·25 Gy in five fractions over 1-2 weeks). Neither participants nor investigators were masked to allocation. Androgen deprivation was not permitted. The primary endpoint of PACE-B is freedom from biochemical or clinical failure. The coprimary outcomes for this acute toxicity substudy were worst grade 2 or more severe Radiation Therapy Oncology Group (RTOG) gastrointestinal or genitourinary toxic effects score up to 12 weeks after radiotherapy. Analysis was per protocol. This study is registered with ClinicalTrials.gov, NCT01584258. PACE-B recruitment is complete and follow-up is ongoing.
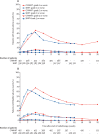
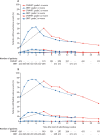
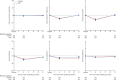
Findings: Between Aug 7, 2012, and Jan 4, 2018, we randomly assigned 874 men to conventionally fractionated or moderately hypofractionated radiotherapy (n=441) or stereotactic body radiotherapy (n=433). 432 (98%) of 441 patients allocated to conventionally fractionated or moderately hypofractionated radiotherapy and 415 (96%) of 433 patients allocated to stereotactic body radiotherapy received at least one fraction of allocated treatment. Worst acute RTOG gastrointestinal toxic effect proportions were as follows: grade 2 or more severe toxic events in 53 (12%) of 432 patients in the conventionally fractionated or moderately hypofractionated radiotherapy group versus 43 (10%) of 415 patients in the stereotactic body radiotherapy group (difference -1·9 percentage points, 95% CI -6·2 to 2·4; p=0·38). Worst acute RTOG genitourinary toxicity proportions were as follows: grade 2 or worse toxicity in 118 (27%) of 432 patients in the conventionally fractionated or moderately hypofractionated radiotherapy group versus 96 (23%) of 415 patients in the stereotactic body radiotherapy group (difference -4·2 percentage points, 95% CI -10·0 to 1·7; p=0·16). No treatment-related deaths occurred.
Interpretation: Previous evidence (from the HYPO-RT-PC trial) suggested higher patient-reported toxicity with ultrahypofractionation. By contrast, our results suggest that substantially shortening treatment courses with stereotactic body radiotherapy does not increase either gastrointestinal or genitourinary acute toxicity.
Funding: Accuray and National Institute of Health Research.
Copyright © 2019 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures

Comment in
-
Stereotactic beam radiotherapy for prostate cancer: is less, more?Lancet Oncol. 2019 Nov;20(11):1471-1472. doi: 10.1016/S1470-2045(19)30652-7. Lancet Oncol. 2019. PMID: 31674307 No abstract available.
-
Optimal patient selection for stereotactic body radiotherapy.Lancet Oncol. 2019 Dec;20(12):e661. doi: 10.1016/S1470-2045(19)30761-2. Lancet Oncol. 2019. PMID: 31797787 No abstract available.
-
Optimal patient selection for stereotactic body radiotherapy - Authors' reply.Lancet Oncol. 2019 Dec;20(12):e662. doi: 10.1016/S1470-2045(19)30747-8. Lancet Oncol. 2019. PMID: 31797788 No abstract available.
-
Increased radiation intensity found to be safe for the treatment of prostate cancer.CA Cancer J Clin. 2020 Mar;70(2):73-74. doi: 10.3322/caac.21595. Epub 2020 Jan 21. CA Cancer J Clin. 2020. PMID: 31961947 No abstract available.
-
Acute toxicities after extremely hypofractionated radiotherapy for prostate cancer: lessons from HYPO-RT-PC and PACE-B.Transl Cancer Res. 2020 Aug;9(8):4469-4472. doi: 10.21037/tcr-20-2061. Transl Cancer Res. 2020. PMID: 35117811 Free PMC article. No abstract available.
References
-
- Cancer Research UK Prostate cancer incidence statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/s...
-
- Government of Canada Canadian cancer statistics: a 2018 special report on cancer incidence by stage. June, 2018. http://www.cancer.ca/~/media/cancer.ca/CW/cancer information/cancer 101/...
-
- American Cancer Society Cancer facts and figures 2018. 2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
-
- National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: prostate cancer. 2016. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf - PubMed
-
- Hamdy FC, Donovan JL, Lane JA. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–1424. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

